| Citation: | Junbo Wang, Sren L Dreyer, Kai Wang, Ziming Ding, Thomas Diemant, Guruprakash Karkera, Yanjiao Ma, Abhishek Sarkar, Bei Zhou, Mikhail V Gorbunov, Ahmad Omar, Daria Mikhailova, Volker Presser, Maximilian Fichtner, Horst Hahn, Torsten Brezesinski, Ben Breitung, Qingsong Wang. P2-type layered high-entropy oxides as sodium-ion cathode materials[J]. Materials Futures, 2022, 1(3): 035104. DOI: 10.1088/2752-5724/ac8ab9 |
In recent years, interest in sodium-ion batteries (SIBs) for various applications, especially large-scale energy storage, has grown [1, 2]. This development is due to the exponential increase in global energy and societal demands for the sustainability of materials [3]. Compared to the widely used lithium-ion batteries (LIBs), SIBs represent a viable alternative because of their lower costs and the use of Na, an abundant resource [4, 5]. In SIB full cells, cathode materials play a crucial role in improving cycling stability and are therefore the subject of many research activities. In general, cathode R&D has focused intensively on layered oxides NaxTMO2 (TM = transition metal) with various structures that offer high theoretical specific capacities, high operating potential and favorable Na+ transport properties [6, 7].
Layered TM oxides are commonly classified into different types, such as O3, P3, P2 and O2, based on the corresponding stacking and coordination of the TMO2 layers, according to the scheme of Delmas et al from the early 1980s [8]. The crystallographic nature of Na-based layered oxides differs from that of Li-based oxides because the Li+ ions prefer different coordination environments. Li-based layered oxides crystallize in so-called O- and T-structures, in which the Li+ ions are located on octahedral (O) and tetrahedral (T) sites [7]. In contrast, Na-based layered oxides generally exist in O3 and P2 structures, with the Na+ ions preferentially occupying the octahedral (O) or prismatic (P) sites. The O3 structure exhibits a high energy barrier for Na+ diffusion, leading to a complex phase transition during sodiation/desodiation [9]. In comparison, the Na+ transport pathways in a trigonal prismatic environment, the P2 structure, allow for faster ion diffusion [10-13]. This is advantageous for energy storage, as it favors the electrochemical reactions and results in larger reversible capacities. However, the structures do not remain stable in the P2 structure, because unfavorable phase transitions between P2 and O2 structures often occur during cycling (caused by layer sliding or TM migration), which affects the reversibility of the reaction due to structural degradation [1, 13, 14]. The phase transition from P2 to O2 occurs at high charge potentials (>4.2 V) when the Na+ ions are extracted from the layer, leading to energetically unfavorable states. Such a transformation is usually not completely reversible and results in a deterioration of the kinetics and lifetime of the electrode [15, 16]. In addition, Mn, an essential component in the layered oxides, plays a non-negligible role in the P2-type materials. During discharge (sodiation) at potentials above 2.0 V, the Mn ions, which are present in the +4 state, are ideally electrochemically inactive and stabilize the structure. However, when the electrode material undergoes further discharge (below 2.0 V), Mn4+ is reduced to Mn3+ [7, 17, 18]. The increased amount of Jahn-Teller active Mn3+ leads to increasing lattice distortion and loss of active material and even results in the appearance of new phases, ultimately resulting in deterioration of the electrochemical properties [18-20]. Therefore, effective strategies to develop high-performance and stable P2 cathodes include, among others, the reduction of unwanted phase transformations and suppression of Mn reduction during cycling, both of which are considered critical for commercial applications [19, 21].
The recently emerging high entropy concept, first introduced in multicomponent metal alloys, opens up new opportunities for materials design and synthesis [22, 23]. In short, by incorporating various elements into a single-phase structure, the large configurational entropy gain may cause entropy-induced structural stabilization and trigger so-called cocktail effects [24-28]. In the field of SIBs, a high-entropy O3-type material has been reported by Zhao et al in 2019, showing a good long-term performance at different charge/discharge rates [29]. More recently, Yang et al reported on a P2-type oxide for SIBs, with the elements being present in equimolar concentrations and improved cycling performance [30]. Despite these examples, this is just the beginning of applying the high entropy concept to layered energy materials. In particular, the role of entropy stabilization and cocktail effects needs further study.
In this work, three different P2-type Na-ion cathode materials for SIBs were synthesized by a simple solid-state reaction method. In the following, Na0.67(Mn0.55Ni0.21Co0.24)O2, Na0.67(Mn0.45Ni0.18Co0.24Ti0.1Mg0.03)O2 and Na0.67(Mn0.45Ni0.18Co0.18Ti0.1Mg0.03Al0.04Fe0.02)O2 are denoted as low-, medium- and high-entropy materials and referred to as 3-NTMO2, 5-NTMO2, and 7-NTMO2, respectively. The oxidation state of Mn is fixed at +4 by tailoring the metal ratio to eliminate the Jahn-Teller effect in the initial state. Operando/post-mortem X-ray diffraction (XRD) and differential electrochemical mass spectrometry (DEMS) were used to gain detailed insights on the stability and to follow the structural evolution of the different samples during cycling. In addition, X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (XAS) and inductively coupled plasma-optical emission spectroscopy (ICP-OES) measurements were conducted to study the behavior of Mn in these materials. In this work, the effect of entropy on the cycling stability, energy density and voltage/capacity retention at two different cut-off potentials is discussed in detail. It is found that 7-NTMO2, with the highest configurational entropy, exhibits the best structural stability and lowest Mn dissolution during cycling. Taken together, the high-entropy strategy is a valuable guide to improve the performance of SIBs and to drive innovation in materials design.
All of the P2-type cathode materials, Na0.67(Mn0.55Ni0.21Co0.24)O2, Na0.67(Mn0.45Ni0.18Co0.24Ti0.1Mg0.03)O2 and Na0.67(Mn0.45Ni0.18Co0.18Ti0.1Mg0.03Al0.04Fe0.02)O2 were synthesized via solid-state reaction. In the first step, stoichiometric amounts of the precursors Na2CO3 (Acros Organics, 99.95%), MgO (abcr GmbH, 99.5%), Al2O3 (Alfa Aesar, 99.5%), TiO2 (Alfa Aesar, 99.5%), Mn3O4 (abcr GmbH, 97%), Fe3O4 (Alfa Aesar, 97%), Co3O4 (Alfa Aesar, 99%) and NiO (Alfa Aesar, 99%) were thoroughly mixed by ball milling at 400 rpm for 2 h and compacted into pellets under 5 t of pressure. After that, the pellets were heated to 500 C for 2 h and calcined at 900 C for 12 h. The final product was obtained by naturally cooling to room temperature and then kept inside an Ar-filled glovebox (O2 < 0.1 ppm, H2O < 0.1 ppm) to prevent further exposure to air.
XRD measurements were conducted on powder samples using a Bruker D8 Advance diffractometer with a Cu-K
For ICP-OES characterization of the as-prepared powder samples, an iCAP 7600 ICP-OES DUO (Thermo Fisher Scientific) was used. More details are provided elsewhere [31, 32].
Scanning electron microscopy (SEM) imaging was performed on a ZEISS Gemini Leo 1530 equipped with an Oxford energy dispersive X-ray spectroscopy (EDX) detector. Transmission electron microscopy (TEM) (with FEI double tilt holder) measurements were carried out on powder samples dispersed on a lacey carbon coated Cu grid. Selected area electron diffraction (SAED) and scanning TEM (STEM)-EDX data were collected using a double-corrected ThermoFisher Themis-Z equipped with a Super-X EDX detector. The TEM microscope was operated at an accelerating voltage of 300 kV. The high-resolution STEM image shown in figure 2(b) and the EDX maps in figure 2(d) were acquired from a thin lamella prepared by focused ion beam milling.
The cathodes contained 70 wt.% oxide as active material, 20 wt.% Super C65 carbon black additive (Imerys Graphite & Carbon) and 10 wt.% Solef 5130 polyvinylidene fluoride (Solvay) binder. The different constituents were uniformly dispersed into N-methyl-2-pyrrolidone using a planetary mixer (Thinky ARE-250). Then, the slurry was cast by doctor blading (100
Operando XRD measurements were conducted on customized CR2032 coin cells with 4 mm diameter Kapton windows on each side using a STOE Stadi P diffractometer equipped with a Ga-Jet X-ray source (Ga-K radiation,
A customized DEMS cell using a 30 mm diameter cathode (3.5 mg cm-2 areal loading) was charged/discharged in a voltage range of 1.5-4.6 V at 20 mA g-1. A 4 mm diameter hole in the center of the cathode allowed for gas flow. GF/D glass microfiber, Na metal and 800
The XPS measurements were performed on a Specs XPS system with a Phoibos 150 energy analyzer using monochromatic Al-K
Operando X-ray absorption near-edge structure (XANES) at the Co K-edge, Ni K-edge and Mn K-edge were performed at the beamline P65 of PETRA III extension of DESY (Hamburg, Germany) in transmission mode. The electrodes were prepared using carbon paper as the current collector and the mass loading of the active material was around 12 mg cm-2. Customized coin cells with Kapton windows were assembled using the same electrolyte as above, which were then connected with an eightfold coin-cell holder coupled with a Bio-Logic VMP3 potentiostat [35]. The cells were cycled at 0.1C (1C = 200 mA g-1) in a voltage range of 1.5-4.6 V. Appropriate metal foils were used for calibration and data was analyzed using the DEMETER package [36].
The Mn content in the electrolyte-filled separators was determined as follows: Each separator was immersed in 5 ml DMC inside an Ar-filled glovebox. The aliquots were then diluted by a factor of 100 with doubly-distilled deionized water and the Mn concentration in the resulting solutions was determined by ICP-OES using an Ultima2 (Horiba).
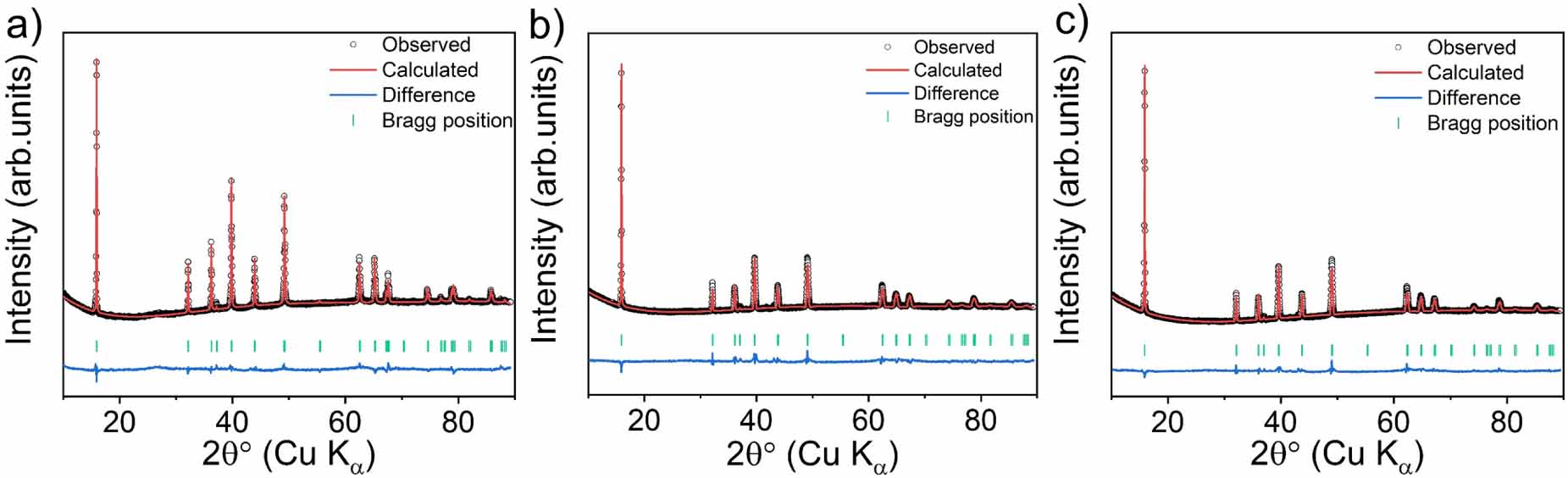
The experimental XRD data and corresponding Rietveld refinements for the 3-NTMO2, 5-NTMO2, and 7-NTMO2 structures are shown in figure 1. All three patterns were analyzed using the P63/mmc space group with the ideal P2 stacking, assuming a random distribution of cations in the TM layers. The refinement results are provided in table S1 of the supporting information. A larger c lattice parameter was calculated for 7-NTMO2 (11.1542(5) ) compared to 3-NTMO2 (11.1343(4) ) and 5-NTMO2 (11.1443(6) ), allowing for a lower diffusion barrier for the Na+ ions. The elemental composition was probed by ICP-OES. As shown in table S2 of the supporting information, the atomic ratios of elements agree well with the targeted compositions. This result indicates that no components were lost during sample preparation. Using equation S1 of the supporting information, the configurational entropy was determined to be 0.99R, 1.34R, and 1.52R for 3-NTMO2, 5-NTMO2, and 7-NTMO2, respectively.
To gain more insights into the structure of the as-prepared materials, the morphology and elemental distribution were investigated by SEM and TEM. Because the samples have similar morphological characteristics, only 7-NTMO2 is shown as an example (figure 2). For comparison, the results for 3-NTMO2 and 5-NTMO2 are presented in figure S1 of the supporting information. As shown in figure 2(a), the powder sample consists of irregular plate-like particles. The size ranges from 1 to 3
The CVs (0.1 mV s-1 scan rate) of electrodes with 3-NTMO2, 5-NTMO2, and 7-NTMO2 are shown in figure 3. A voltage range between 1.5 V and 4.6 V vs. Na+/Na was applied to observe the evolution of peaks at 4.2 V associated with both phase transition and extraction of Na+ [15, 17, 37]. When charged above 4.2 V, a further decrease in sodium content in the P2 structure leads to an energetically favored misalignment of oxygen atoms along the c-axis, and part of the capacity is attributed to oxygen redox (see the section on DEMS for more details) [13, 38-40]. Therefore, in the first two cycles, the oxidation reaction peaks (anodic sweep) at about 4.3 V shift toward lower potential and show a decrease in intensity.
Shifts of different strength of the redox peaks above 3.5 V were observed for these three electrode materials. The changes are most pronounced for 3-NTMO2, indicating that the structure of this material changes more strongly upon cycling than for 5-NTMO2 and 7-NTMO2. 3-NTMO2 exhibits intense phase transitions during the initial cycles (see also the section on operando XRD below). In the following cycles, the potential of the reduction reaction (cathodic sweep) decreases to 3.7 V in the fourth cycle. For 5-NTMO2, both the intensity drop and position change of the redox peak at 4.3 V are not as pronounced as for 3-NTMO2, which can be explained by improved structural stability and lower polarization. Compared to 3-NTMO2 and 5-NTMO2, the 7-NTMO2 electrode shows the best reversibility in the high-voltage range (3.8-4.6 V) throughout testing. The reduction of Mn4+ to Mn3+ and the associated Jahn-Teller distortion are most evident for 3-NTMO2 in the low-voltage range (about 2.2 V). This process can induce a phase transition from P2 to P2ʹ (space group Cmcm or C2/n) at high Na+ content, as reported by Benoit et al and Hasa et al [38, 41-43]. This in turn leads to partial dissolution of Mn and a decrease in capacity. As shown in figure 3(a), the intensity and shift (polarization) of the Mnn+ redox reaction peaks gradually increase with continuous cycling of 3-NTMO2, indicating that the corresponding Mnn+ redox contributes to capacity loss [17, 44]. This behavior is less pronounced for 5-NTMO2 (figure 3(b)). For 7-NTMO2 (figure 3(c)), the intensity of the Mnn+ redox peaks barely change during the first four cycles. These results demonstrate the good reversibility of the high-entropy material 7-NTMO2 compared with the other two P2 materials.
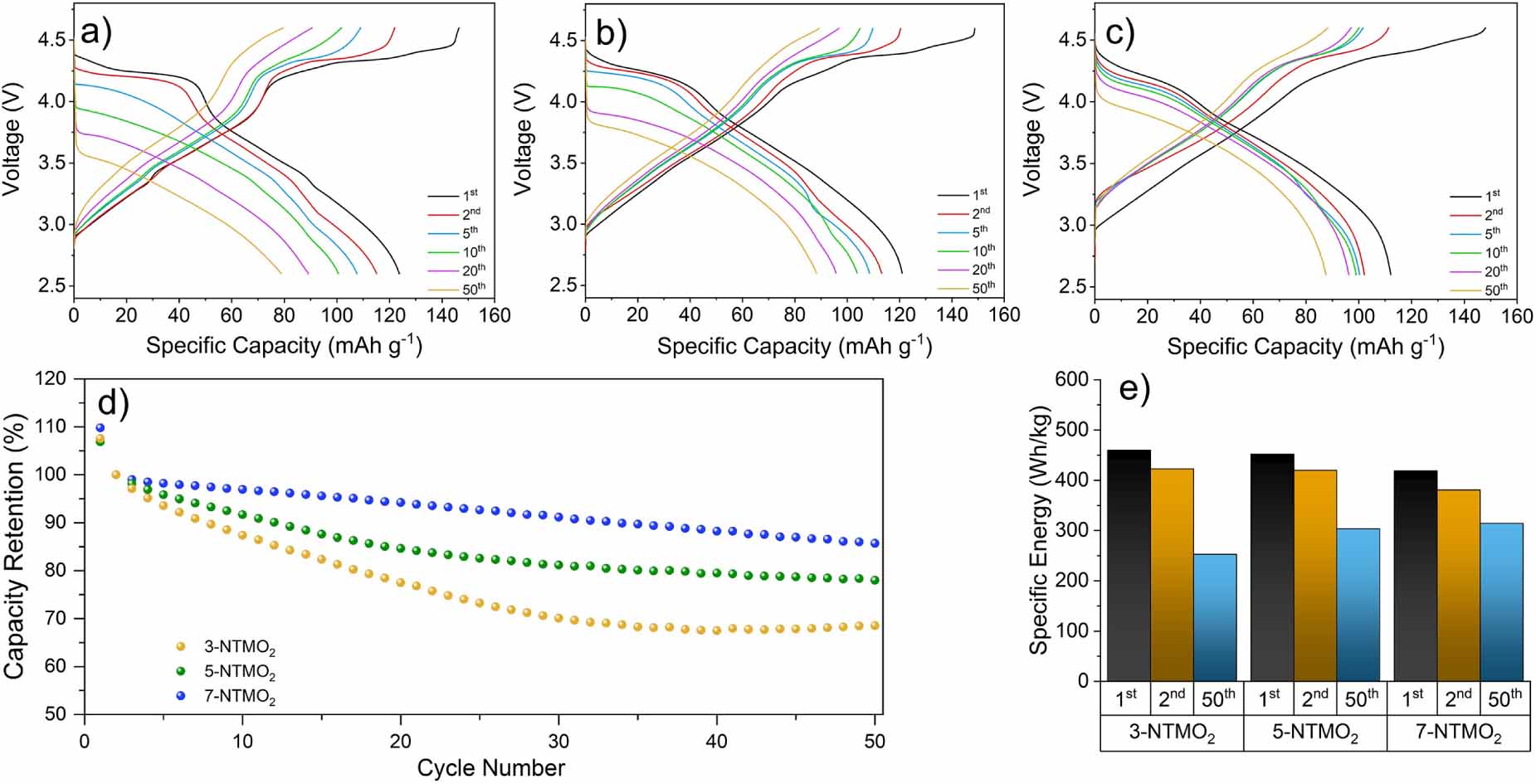
Galvanostatic cycling experiments were performed to further investigate the electrochemical performance. For activation, the 1st cycle was performed at a rate of 0.05C. The charge-discharge profiles of the 2nd, 5th, 10th, 20th and 50th cycles at 0.5C in two different potential ranges were analyzed. The electrochemical properties of the electrodes were characterized in a voltage range of 2.6-4.6 V (figure 4) firstly, so that the formation of Mn3+ can be excluded. The 3-NTMO2 delivers a specific discharge capacity of 115 mA h g-1 in the 2nd cycle, which is slightly higher than that of 5-NTMO2 (113 mA h g-1) and 7-NTMO2 (102 mA h g-1), due to a higher content of electrochemically active elements (Co, Ni, and Mn). Normally, distinct plateaus starting at about 4.2 V indicate the occurrence of two-phase reactions (the P2-O2 phase transition) during the charging process (figures 4(a) and (b)) [17, 44-46]. In the case of 7-NTMO2, a smooth charging curve indicates the extraction of sodium ions via solid-solution reaction [16, 21, 30].
Capacity loss and voltage decay were observed for all three materials, although to different extents. A sharp voltage drop is evident from the charge/discharge profile of 3-NTMO2. The midpoint voltage (MPV) of the discharge drops from 3.72 V at the 2nd cycle to 3.23 V at the 50th cycle. The sharp drop is consistent with the intensity decrease and shift of the reduction peaks to lower potentials in the above-discussed CV data. In contrast, the MPV for 5-NTMO2 and 7-NTMO2 decreases from 3.78 V to 3.51 V and from 3.78 V to 3.67 V, respectively. Therefore, 7-NTMO2 exhibits the best discharge voltage retention.
Figure 4(d) shows the cycling performance of the materials at 0.5C rate. Although a larger initial capacity is achieved, the 3-NTMO2 and 5-NTMO2 electrodes reveal significant fading over the first 15 cycles. Thereafter, the trend toward decreasing capacity is not mitigated for 3-NTMO2, while it is more moderate for 5-NTMO2. In contrast, the 7-NTMO2 electrode exhibits superior cycling stability (see also specific capacities and Coulombic efficiencies with some discussion in figure S2 of the supporting information). Ultimately, at the 50th cycle, 69%, 78% and 86% of the initial capacity was retained for 3-NTMO2, 5-NTMO2, and 7-NTMO2, respectively. The observed voltage drops and capacity losses lead to a degradation in specific energy of the active materials. Translating these capacity values into gravimetric energy densities with a theoretical Na metal anode (figure 4(e)), the second discharge provides specific energies of 423, 420, and 381 W h kg-1 and the 50th cycle results in specific energies of 253, 303, and 314 W h kg-1 for 3-NTMO2, 5-NTMO2, and 7-NTMO2, respectively.
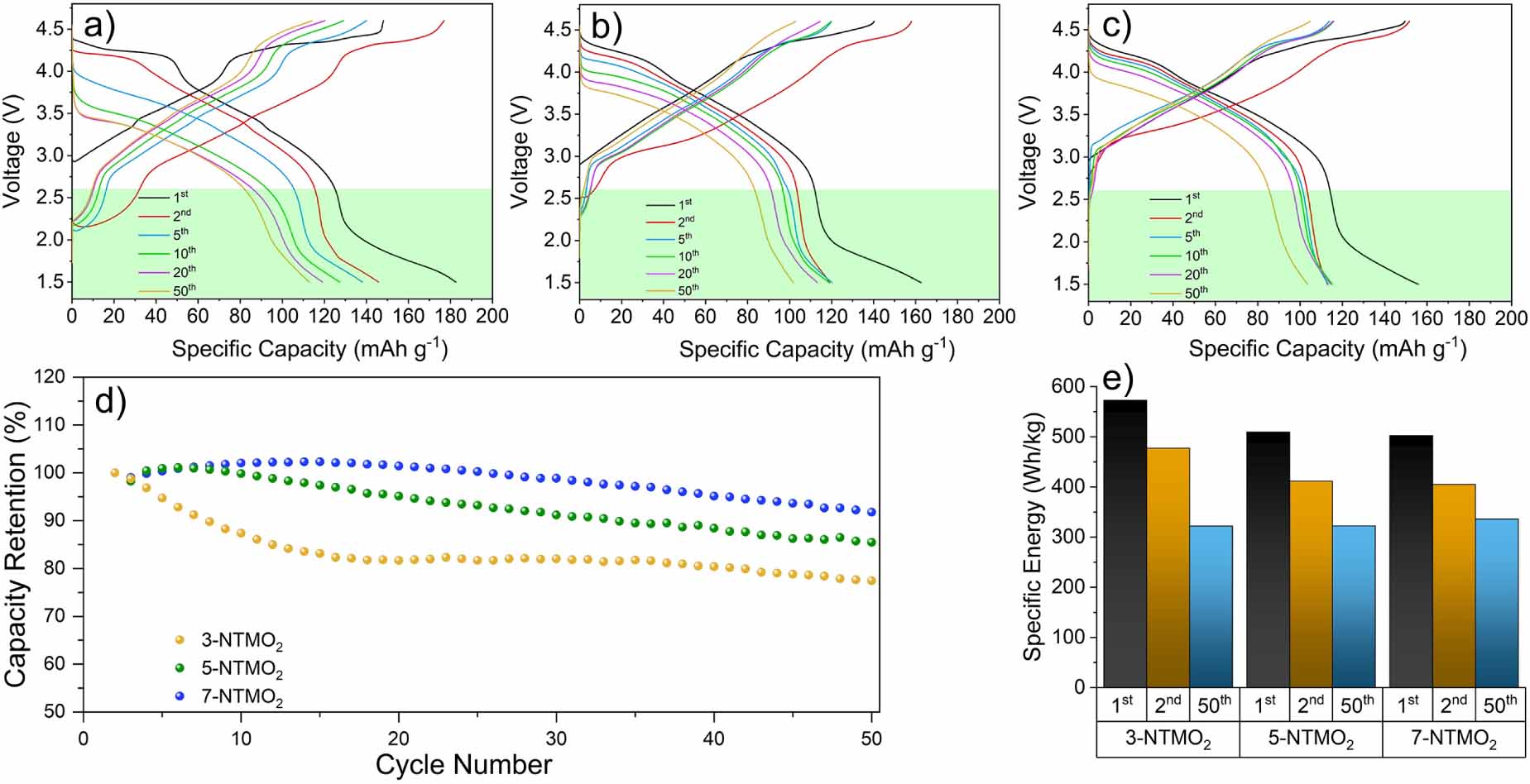
Figure 5 shows the charge-discharge profiles in a voltage range of 1.5-4.6 V. A wider range was chosen to probe the influence of structural rearrangement with Mnn+ redox on the electrochemical performance [17, 47, 48]. Disproportionation reactions of Mn3+ ions can produce soluble Mn2+ ions, leading to a steady loss in active material [17]. After the second cycle, the specific charge capacity was 146, 119, and 113 mA h g-1 for 3-NTMO2, 5-NTMO2, and 7-NTMO2, respectively. The differences in discharge capacities (31, 6, and 11 mA h g-1) are due to the insertion of Na+ ions below 2.6 V, reaching the theoretical Na+ intercalation limit at which the active elements are in a low-valence state. This will be discussed in more detail in the section on XAS. The reduction reaction involving the Mn4+/Mn3+ redox couple occurs at about 2.2 V (highlighted by green boxes in figures 5(a)-(c)) in the discharge process. The slopes are steeper for 5-NTMO2 and 7-NTMO2 than for 3-NTMO2, indicating a lower degree of Mnn+ redox [17, 49]. For 3-NTMO2, the voltage drop and capacity loss are more pronounced than in the narrow potential window (2.6-4.6 V, see figure 4). Although good reversibility is observed between the 20th and the 50th cycles, the strong polarization and performance degradation compared to the first cycles cannot be ignored. These results suggest that the disproportionation of Mn in the discharged state may have a significant impact on the electrode material in the initial cycles [50, 51]. As shown in figure 5(d), a similar trend in terms of capacity degradation can be observed as for the 2.6-4.6 V range. However, after 50 cycles, the cell with 3-NTMO2 is capable of delivering a larger specific capacity (113 mA h g-1) than 5-NTMO2 and 7-NTMO2. This is likely because of the improved discharge capacity caused by the Mn4+/Mn3+ reduction reactions below 2.6 V. Nevertheless, the rapid capacity decay leads to a low capacity retention of 61.9% for 3-NTMO2. In contrast, the 5-NTMO2 and 7-NTMO2 electrodes show a reduced capacity loss with remaining specific capacities of 102 and 104 mA h g-1 after 50 cycles, corresponding to 85.5% and 91.8%, respectively (see also specific capacities and Coulombic efficiencies with some discussion in figure S2 of the supporting information). Accordingly, the specific discharge energy in the second cycle is 477, 411 and 405 W h kg-1 for 3-NTMO2, 5-NTMO2, and 7-NTMO2, respectively, and decreases to 322, 322, and 336 W h kg-1 in the 50th cycle (figure 5(e)). These results show that 7-NTMO2 exhibits a significantly improved cycling performance at higher potentials as well as below 2.6 V due to increased structural stability, thereby proving that the high-entropy concept positively affects the charge-storage properties.
The strong capacity degradation observed over the first two cycles can probably be attributed to phase transitions, which were examined by operando XRD. XRD patterns were recorded in a voltage range of 1.5-4.6 V. The corresponding 2D contour plots are shown in figure 6. For 3-NTMO2 (figure 6(a)), the (002) reflection at 12.2 2
During the 2nd cycle, the intensity of the (002) reflection decreases dramatically compared to the region above 4.2 V during the 1st cycle. Nevertheless, the P2 (002) reflection at 12.2 2 shifts to higher angles with the presence of the O2 (002) reflection at 13.3 2
In contrast to 3-NTMO2 and 5-NTMO2, the structural evolution of 7-NTMO2 is slightly different when charged above 4.35 V (figure 6(c)). The P2 (002) reflection only shifts marginally to higher angles (with a maximum of 13.1 2
The reaction mechanism (charge compensation process) during the initial charge/discharge cycle in a voltage range of 1.5-4.6 V was further studied by operando XAS in transmission mode. XANES spectra collected at the Ni K-edge (8333 eV) and the Co K-edge (7709 eV) are shown in figure 7. Figures 7(a)-(c) shows the Ni K-edge XANES spectra for 3-NTMO2, 5-NTMO2 and 7-NTMO2, respectively. The oxidation state of Ni was determined from the half-height energy position. Comparing the XAS data of the pristine cathode materials to the standard reference NiO, it is found that they contain predominantly Ni2+. During the charging process (until 4.6 V, scan labeled as charged), the edge shifts continuously to higher energies (by about 4 eV), demonstrating the oxidation of Ni2+ to Ni4+. During discharge, the Ni-K edge shows hardly any shift before reaching a potential of about 4 V, followed by the 4 eV shift, thus indicating the fully reversible reduction back to Ni2+. Overall, all three materials show a similar Ni contribution to the electrochemical redox reactions. Similarly, the Co K-edge XANES spectra (figures 7(e)-(f)) reveal the reversible redox reaction of Co3+ (with close energy shift during charge and discharge). Because the electrochemically active Ni and Co contribute to the overall capacity in the same way, the lower capacity achieved with 7-NTMO2 can be attributed to the lower overall fractions of Ni and Co in the material.
For an overview of gas evolution in SIBs, the reader is referred to a study by Zhang et al [58]. The gas evolution behavior of the different cathode materials was investigated using DEMS. For this purpose, the cells were cycled at a specific current of 20 mA g-1 in a custom setup for two consecutive cycles. Figures 8 and S4 of the supporting information show the correlation between the voltage profiles and the gas evolution rates for the most evolved gases, hydrogen (H2, m/z = 2) and carbon dioxide (CO2, m/z = 44). The origin of these gases can be inferred from the analogy with gas evolution in LIBs, where CO2 can originate either from chemical oxidation of the electrolyte associated with the release of lattice oxygen or from electrochemical oxidation of the electrolyte (or, mainly during the first cycle, from surface carbonates) [39, 59-62]. Since the released O2 in the case of layered oxide cathode materials for LIBs is apparently highly reactive in nature, it is rarely detected directly as molecular oxygen, but indirectly as the reaction product CO2 [63, 64]. In this work, the same observation is made: while O2 evolution is below the quantitation limit, large amounts of CO2 are clearly produced. H2 is formed by the reduction of trace amounts of water and alcohol at the anode. Its release at high potentials is usually explained by the formation of protic species as byproducts of electrolyte oxidation reactions at the cathode and their subsequent migration to the anode, where they are reduced [65].
In the first cycle, all three materials show strong gas evolution, see figure S4 of the supporting information. The highest rate of evolution of CO2 was not at the highest potential, as expected, but during the P2-O2 phase transition at the 4.3 V plateau. It should be noted that residual carbonates can often explain much of the gas evolution in the initial cycle [59]. However, no carbonate bands are visible in the Fourier transform infrared spectra of the materials (figure S5 of the supporting information), indicating a carbonate-free surface. A similar pattern is also observed in the H2 evolution rates, suggesting electrolyte oxidation as the cause of gas evolution. Because gas release is irreversible, the dq/dV plots (figure S6 of the supporting information) do not show a corresponding cathodic peak during discharge.
From these results, it can be concluded that at about 4.3 V, significant amounts of lattice oxygen are released during the phase transition, which reacts with the electrolyte, forming CO2 by chemical oxidation. A similar observation has been made for Mn-rich cathode materials for LIBs and confirmed by isotope labeling experiments [66]. As an example, for the P2-type cathode material Na0.78(Li0.25Ni0.75)O2, isotope labeling also confirmed a significant loss of lattice oxygen by electrolyte oxidation to CO2, but at a potential of 5.0 V [67]. As with these materials, the intense gas evolution in the first cycle may indicate the presence of anion redox in the materials studied, which has been reported for several SIB cathode materials [68, 69]. The presence, extent and reversibility of anion redox is beyond the scope of this work and will be investigated in a follow-up study.
At lower potentials toward the end of discharge, additional CO2 evolution, but almost no H2 evolution, is observed (figure S7 of the supporting information). Because two peaks are visible, one after the rapid voltage drop from 3.0 to 2.0 V and another at the end of discharge below 1.8 V, two different mechanisms can be assumed. By applying constant voltage steps, the peaks can be disentangled. The first peak coincides with the Mn4+/3+ redox and Mn disproportionation. The gas evolution can be attributed to the cathode-solid electrolyte interphase and solid electrolyte interphase damage caused by Mn2+ [70]. Continuous CO2 evolution is observed at potentials below 1.7 V, which can most likely be explained by the reduction of FEC at the cathode side, which has already been reported to occur from 1.8 V vs. Li+/Li with CO2 evolution [71]. The observation of increasing steady-state currents at the end of each constant voltage step argues for an electrochemical reaction pathway, e.g. FEC reduction.
The gas evolution at higher potentials during the second cycle is shown in figure 8. A clear difference in behavior between the three materials can be observed here. As the length of the voltage plateau decreases, the gas evolution profiles also change, with 3-NTMO2 having both the most distinct plateau and gas evolution shoulder. In the dq/dV plot (figure S6 of the supporting information), 3-NTMO2 is also the only material that still exhibits an irreversible shoulder at 4.3 V in the second cycle. Figure S8 of the supporting information shows a comparison of the CO2 evolution of all materials during the second charge as a function of the specific charge capacity, and figure S9 of the supporting information displays the CO2 evolution as a function of the voltage. However, due to the steep profiles between 3.8 V and 4.2 V, the initial voltage of the gas evolution cannot be accurately determined. Here, an exponential trend is observed [33]. Previously, it was shown that significant electrochemical oxidation of the electrolyte in LIBs only occurs above 5.0 V vs. Li+/Li, corresponding to 4.67 V vs. Na+/Na [39]. From the observed onset of gas evolution already around 4.0 V and the clear shoulder peak in the H2 evolution of 3-NTMO2 (see figures 8 and S4 of the supporting information), it can be concluded that mainly chemical oxidation takes place, caused by the irreversible loss of lattice oxygen [66].
Table S3 of the supporting information summarizes the specific capacities and total amounts of gases released for all three materials. It can be concluded that increased CO2 evolution due to enhanced release of lattice oxygen explains, at least in part, the higher irreversible capacity and lower capacity retention during long-term cycling (figure 5(d)) of 3-NTMO2 compared to both 5-NTMO2 and 7-NTMO2. While the release of lattice oxygen depends on the degree of desodiation, and therefore on the specific charge capacity, the difference in gas evolution is not observed at the end of charge, but when the plateau is reached around 4.3 V. At the same time, the main difference in specific charge capacity is due to charging at low potentials between 1.5 V and 2.6 V.
In summary, DEMS analysis shows that the gas evolution behavior changes with increasing configurational entropy and the total amount of evolved gas is reduced. Since the lattice oxygen is the major source of O2, increasing the configurational entropy reduces the detrimental release of lattice oxygen, partially explaining the better material performance.
To further investigate the structural evolution during long-term cycling and verify the superior structural stability of 7-NTMO2, ex situ XRD measurements were performed prior to cycling and after the 50th cycle in the discharged state (figure S10 of the supporting information). The main reflections of the cathode materials can still be assigned to the P2 structure. However, different degrees of degradation are evident from the data. For 3-NTMO2, the XRD pattern shows a strong shift of the (002) and (004) reflections to lower angles. The increased c-axis lattice parameter indicates irreversible expansion of the material along the direction perpendicular to the plane of the Na+ layers, thus suggesting incomplete sodiation [57, 72, 73]. Note that incomplete sodiation as a result of deteriorated diffusion kinetics could be due to the TM dissolution, leading to a change in chemical environment of the oxygen atoms. In comparison, the reflection shifts are much less pronounced for 5-NTMO2, while the 7-NTMO2 electrode shows the best structural stability after 50 cycles. Moreover, the full-width at half maximum of the (002) and (004) reflections of 3-NTMO2 increased more strongly than for 5-NTMO2 and 7-NTMO2. This increase is indicative of a decrease in crystallinity of the material during cycling, which is often associated with the loss of Na+ intercalation sites and structural irreversibilities [72, 74].
As described above, the Mnn+ redox for the larger voltage range (1.5-4.6 V) leads to unfavorable Mn dissolution and consequently to a decrease in capacity. The changes in oxidation state of Mn during cycling were also investigated by operando XAS. Figures 9(a)-(c) shows the normalized Mn K-edge (6539 eV) XANES spectra during the initial charge/discharge cycle for electrodes with 3-NTMO2, 5-NTMO2, and 7-NTMO2, respectively. Compared to the standard reference MnO2, Mn is mainly in +4 state in all three cathode materials in the pristine state. This is consistent with the XPS results shown in figure S11 of the supporting information. The pristine materials show a peak doublet at 642.6 eV (Mn 2p3/2) and 654.3 eV (Mn 2p1/2), which can be attributed to Mn4+. For 3-NTMO2, a clear shift of the Mn edge toward lower energy is observed after the first discharge cycle, demonstrating the overall decrease in Mn oxidation state (presence of Mn3+) compared to the pristine state. In contrast, there is no perceptible change in the XANES spectra of 5-NTMO2 and 7-NTMO2.
Finally, ICP-OES was used to quantify the degree of Mn dissolution into the electrolyte. After 50 cycles between 1.5 and 4.6 V, the electrolyte was harvested from the coin cells. The data in figure 9(d) show that Mn dissolution decreases progressively from 3-NTMO2 to 7-NTMO2, i.e. with increasing configurational entropy. It is worth mentioning that the ICP-OES data agree with the XPS results for the oxidation state of Mn after 50 cycles. Taken together, it seems that the stronger Mn dissolution is a contributing factor to the poorer cycling stability of 3-NTMO2. In addition, the results confirm that 7-NTMO2 with the highest configurational entropy exhibits greatly improved structural stability.
Three different layered P2-type oxides, denoted as low-, medium- and high-entropy oxides, were successfully synthesized via solid-state reaction and investigated as cathode materials for SIBs. The high-entropy 7-NTMO2 was found to exhibit superior reversibility during sodiation/desodiation in the potential ranges of 2.6-4.6 V and 1.5-4.6 V. In contrast, rapid capacity fading was observed in the case of low-entropy 3-NTMO2 and moderate decay for the medium-entropy 5-NTMO2. By combining operando and ex situ XRD, it was found that all materials tend to undergo a solid-solution reaction after several cycles, accompanied by a weakening of the P2 O2 phase transition. The high configurational entropy is beneficial to mitigate the phase transition and maintain structural stability. Furthermore, DEMS analysis showed a decrease in gas release with increasing configurational entropy. At the same time, the Mn disproportionation at low potentials, leading to the formation of soluble Mn2+, was suppressed in 7-NTMO2, as demonstrated by XPS and XAS and confirmed by ICP-OES. As the contribution of the high-entropy effect to the cycling performance attracts increasing attention, we provide here further compelling and encouraging evidence for the potential of high-entropy materials for electrochemical energy-storage applications.
J W, K W, Z D and B Z acknowledge financial support from the China Scholarship Council (CSC). G K and M F gratefully acknowledge financial support by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy, EXC 2154, project number 390874152. A O acknowledges financial support from the Federal Ministry of Education and Research (Bundesministerium fr Bildung und Forschung, BMBF) under the project KaSiLi’ (03XP0254D) in the competence cluster ExcellBattMat’. H H, B B and T B acknowledge financial support from the Helmholtz Association (DigiBat project). Financial support by the German Research Foundation (to H H, Grant No. HA 1344/43-1) is gratefully acknowledged. Q W and B B acknowledge the support from EnABLES and EPISTORE, projects funded by the European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 730957 and 101017709, respectively. B B also acknowledges funding from the Kera-Solar project (Carl Zeiss Foundation). Beamtime allocation and support at beamline P65 of the PETRA III synchrotron (Deutsches Elektronen-Synchrotron DESY, Hamburg, Germany) is gratefully acknowledged. The INM authors thank Eduard Arzt (INM) for his continuing support. The authors acknowledge Andrea Jung (INM) for her support on ICP-OES measurements. The authors further acknowledge the support from the Karlsruhe Nano Micro Facility (KNMF, www.knmf.kit.edu), a Helmholtz research infrastructure at Karlsruhe Institute of Technology (KIT, www.kit.du).
Conflict of interest
There are no conflicts to declare.
Author contributions
J W, B B and Q W conceived the idea and designed the experiments. B B, H H and Q W supervised the project. S L D and T B conducted the DEMS measurements and analysed the data. K W and Z D performed the TEM measurements and analysed the data. T D, G K and M F collected the XPS spectra and analyzed the data. A S and Y M provided valuable suggestions during the whole project. B Z, M V G, A O and D M helped with the XAS measurements and analyzed the data. V P helped with the ICP-OES measurements. J W, B B and Q W prepared the initial manuscript. All authors discussed the results and contributed to discussion and reviewing the manuscript.
Present address: Bavarian Center for Battery Technology (BayBatt), University of Bayreuth, Universittsstrasse 30, 95447 Bayreuth, Germany and Department of Chemistry, University of Bayreuth, Universittsstrasse 30, 95447 Bayreuth, Germany.
| [1] |
Hwang J-Y, Myung S-T, Sun Y-K 2017 Sodium-ion batteries: present and future Chem. Soc. Rev. 46 3529-614 DOI: 10.1039/c6cs00776g
|
| [2] |
Delmas C 2018 Sodium and sodium-ion batteries: 50 years of research Adv. Energy Mater. 8 1703137 DOI: 10.1002/aenm.201703137
|
| [3] |
Tarascon J-M 2020 Na-ion versus Li-ion batteries: complementarity rather than competitiveness Joule 4 1616-20 DOI: 10.1016/j.joule.2020.06.003
|
| [4] |
Palomares V, Casas-Cabanas M, Castillo-Martnez E, Han M H, Rojo T 2013 Update on Na-based battery materials. A growing research path Energy Environ. Sci. 6 2312-37 DOI: 10.1039/c3ee41031e
|
| [5] |
Delmas C, Carlier D, Guignard M 2021 The layered oxides in lithium and sodium-ion batteries: a solid-state chemistry approach Adv. Energy Mater. 11 2001201 DOI: 10.1002/aenm.202001201
|
| [6] |
Han M H, Gonzalo E, Singh G, Rojo T 2015 A comprehensive review of sodium layered oxides: powerful cathodes for Na-ion batteries Energy Environ. Sci. 8 81-102 DOI: 10.1039/C4EE03192J
|
| [7] |
Clment R J, Bruce P G, Grey C P 2015 Reviewmanganese-based P2-type transition metal oxides as sodium-ion battery cathode materials J. Electrochem. Soc. 162 A2589-604 DOI: 10.1149/2.0201514jes
|
| [8] |
Delmas C, Fouassier C, Hagenmuller P 1980 Structural classification and properties of the layered oxides Physica B+C 99 81-85 DOI: 10.1016/0378-4363(80)90214-4
|
| [9] |
Hwang J-Y, Yoon C S, Belharouak I, Sun Y-K 2016 A comprehensive study of the role of transition metals in O3-type layered Na[NixCoyMnz]O2x = 1/3, 0.5, 0.6, and 0.8) cathodes for sodium-ion batteries J. Mater. Chem. A 4 17952-9 DOI: 10.1039/C6TA07392A
|
| [10] |
Wang Y, Xiao R, Hu Y-S, Avdeev M, Chen L 2015 P2-Na0.6[Cr0.6Ti0.4]O2 cation-disordered electrode for high-rate symmetric rechargeable sodium-ion batteries Nat. Commun. 6 6954 DOI: 10.1038/ncomms7954
|
| [11] |
T-Y Y, Hwang J-Y, Aurbach D, Sun Y-K 2017 Microsphere Na0.65[Ni0.17Co0.11Mn0.72]O2 cathode material for high-performance sodium-ion batteries ACS Appl. Mater. Interfaces 9 44534-41 DOI: 10.1021/acsami.7b15267
|
| [12] |
Katcho N A, Carrasco J, Saurel D, Gonzalo E, Han M, Aguesse F, Rojo T 2017 Origins of bistability and Na ion mobility difference in P2- and O3-Na2/3Fe2/3Mn1/3O2 cathode polymorphs Adv. Energy Mater. 7 1601477 DOI: 10.1002/aenm.201601477
|
| [13] |
Zhao C, et al 2020 Revealing high Na-content P2-type layered oxides as advanced sodium-ion cathodes J. Am. Chem. Soc. 142 5742-50 DOI: 10.1021/jacs.9b13572
|
| [14] |
Lyu Y, Liu Y, Yu Z-E, Su N, Liu Y, Li W, Li Q, Guo B, Liu B 2019 Recent advances in high energy-density cathode materials for sodium-ion batteries Sustain. Mater. Technol. 21 e00098 DOI: 10.1016/j.susmat.2019.e00098
|
| [15] |
Lee D H, Xu J, Meng Y S 2013 An advanced cathode for Na-ion batteries with high rate and excellent structural stability Phys. Chem. Chem. Phys. 15 3304-12 DOI: 10.1039/c2cp44467d
|
| [16] |
Zhang J, Wang W, Wang W, Wang S, Li B 2019 Comprehensive review of P2-type Na2/3Ni1/3Mn2/3O2, a potential cathode for practical application of Na-Ion batteries ACS Appl. Mater. Interfaces 11 22051-66 DOI: 10.1021/acsami.9b03937
|
| [17] |
Liu Q, Hu Z, Chen M, Zou C, Jin H, Wang S, Gu Q, Chou S 2019 P2-type Na2/3Ni1/3Mn2/3O2 as a cathode material with high-rate and long-life for sodium ion storage J. Mater. Chem. A 7 9215-21 DOI: 10.1039/C8TA11927A
|
| [18] |
Liu T, et al 2019 Correlation between manganese dissolution and dynamic phase stability in spinel-based lithium-ion battery Nat. Commun. 10 4721 DOI: 10.1038/s41467-019-12626-3
|
| [19] |
Kumakura S, Tahara Y, Kubota K, Chihara K, Komaba S 2016 Sodium and manganese stoichiometry of P2-type Na2/3MnO2 Angew. Chem., Int. Ed. Engl. 55 12760-3 DOI: 10.1002/anie.201606415
|
| [20] |
Zhan C, Wu T, Lu J, Amine K 2018 Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodesa critical review Energy Environ. Sci. 11 243-57 DOI: 10.1039/C7EE03122J
|
| [21] |
Zuo W, et al 2020 Highly-stable P2-Na0.67MnO2 electrode enabled by lattice tailoring and surface engineering Energy Storage Mater. 26 503-12 DOI: 10.1016/j.ensm.2019.11.024
|
| [22] |
Cantor B, Chang I T H, Knight P, Vincent A J B 2004 Microstructural development in equiatomic multicomponent alloys Mater. Sci. Eng. A 375-377 213-8 DOI: 10.1016/j.msea.2003.10.257
|
| [23] |
Yeh J-W, Chen S-K, Lin S-J, Gan J-Y, Chin T-S, Shun T-T, Tsau C-H, Chang S-Y 2004 Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes Adv. Eng. Mater. 6 299-303 DOI: 10.1002/adem.200300567
|
| [24] |
Rost C M, Sachet E, Borman T, Moballegh A, Dickey E C, Hou D, Jones J L, Curtarolo S, Maria J-P 2015 Entropy-stabilized oxides Nat. Commun. 6 8485 DOI: 10.1038/ncomms9485
|
| [25] |
Sarkar A, et al 2018 High entropy oxides for reversible energy storage Nat. Commun. 9 3400 DOI: 10.1038/s41467-018-05774-5
|
| [26] |
Ma Y, Ma Y, Wang Q, Schweidler S, Botros M, Fu T, Hahn H, Brezesinski T, Breitung B 2021 High-entropy energy materials: challenges and new opportunities Energy Environ. Sci. 14 2883-905 DOI: 10.1039/D1EE00505G
|
| [27] |
Ma Y, et al 2021 High-entropy metal-organic frameworks for highly reversible sodium storage Adv. Mater. 33 2101342 DOI: 10.1002/adma.202101342
|
| [28] |
Wang Q, Velasco L, Breitung B, Presser V 2021 Highentropy energy materials in the age of big data: a critical guide to nextgeneration synthesis and applications Adv. Energy Mater. 11 2102355 DOI: 10.1002/aenm.202102355
|
| [29] |
Zhao C, Ding F, Lu Y, Chen L, Hu Y-S 2020 High-entropy layered oxide cathodes for sodium-ion batteries Angew. Chem., Int. Ed. Engl. 59 264-9 DOI: 10.1002/anie.201912171
|
| [30] |
Yang L, Chen C, Xiong S, Zheng C, Liu P, Ma Y, Xu W, Tang Y, Ong S P, Chen H 2021 Multiprincipal component P2-Na0.6(Ti0.2Mn0.2Co0.2Ni0.2Ru0.2O2 as a high-rate cathode for sodium-ion batteries JACS Au 1 98-107 DOI: 10.1021/jacsau.0c00002
|
| [31] |
Wang Q, et al 2019 Multi-anionic and -cationic compounds: new high entropy materials for advanced Li-ion batteries Energy Environ. Sci. 12 2433-42 DOI: 10.1039/C9EE00368A
|
| [32] |
Wang J, et al 2020 Lithium containing layered high entropy oxide structures Sci. Rep. 10 18430 DOI: 10.1038/s41598-020-75134-1
|
| [33] |
Berkes B B, Jozwiuk A, Sommer H, Brezesinski T, Janek J 2015 Simultaneous acquisition of differential electrochemical mass spectrometry and infrared spectroscopy data for in situ characterization of gas evolution reactions in lithium-ion batteries Electrochem. Commun. 60 64-69 DOI: 10.1016/j.elecom.2015.08.002
|
| [34] |
Berkes B B, Jozwiuk A, Vraar M, Sommer H, Brezesinski T, Janek J 2015 Online continuous flow differential electrochemical mass spectrometry with a realistic battery setup for high-precision, long-term cycling tests Anal. Chem. 87 5878-83 DOI: 10.1021/acs.analchem.5b01237
|
| [35] |
Herklotz M, et al 2016 A novel high-throughput setup for in situ powder diffraction on coin cell batteries J. Appl. Crystallogr. 49 340-5 DOI: 10.1107/S1600576715022165
|
| [36] |
Ravel B, Newville M 2005 ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT J. Synchrotron Radiat. 12 537-41 DOI: 10.1107/S0909049505012719
|
| [37] |
Li J, et al 2019 P2Type Na0.67Mn0.8Cu0.1Mg0.1O2 as a new cathode material for sodium-ion batteries: insights of the synergetic effects of multi-metal substitution and electrolyte optimization J. Power Sources 416 184-92 DOI: 10.1016/j.jpowsour.2019.01.086
|
| [38] |
Hasa I, Passerini S, Hassoun J 2017 Toward high energy density cathode materials for sodium-ion batteries: investigating the beneficial effect of aluminum doping on the P2-type structure J. Mater. Chem. A 5 4467-77 DOI: 10.1039/C6TA08667E
|
| [39] |
Jung R, Metzger M, Maglia F, Stinner C, Gasteiger H A 2017 Chemical versus electrochemical electrolyte oxidation on NMC111, NMC622, NMC811, LNMO, and conductive carbon J. Phys. Chem. Lett. 8 4820-5 DOI: 10.1021/acs.jpclett.7b01927
|
| [40] |
Risthaus T, et al 2018 A high-capacity P2 Na2/3Ni1/3Mn2/3O2 cathode material for sodium ion batteries with oxygen activity J. Power Sources 395 16-24 DOI: 10.1016/j.jpowsour.2018.05.026
|
| [41] |
Mortemard de Boisse B, Carlier D, Guignard M, Bourgeois L, Delmas C 2014 P2-NaxMn1/2Fe1/2O2 phase used as positive electrode in Na batteries: structural changes induced by the electrochemical (De)intercalation process Inorg. Chem. 53 11197-205 DOI: 10.1021/ic5017802
|
| [42] |
Wang J, et al 2020 Insights into P2-type layered positive electrodes for sodium batteries: from long- to short-range order ACS Appl. Mater. Interfaces 12 5017-24 DOI: 10.1021/acsami.9b18109
|
| [43] |
Liu Z, Xu X, Ji S, Zeng L, Zhang D, Liu J 2020 Recent progress of P2-Type layered transition-metal oxide cathodes for sodium-ion batteries Chemistry A 26 7747-66 DOI: 10.1002/chem.201905131
|
| [44] |
Z-Y L, Gao R, Sun L, Hu Z, Liu X 2015 Designing an advanced P2-Na0.67Mn0.65Ni0.2Co0.15O2 layered cathode material for Na-ion batteries J. Mater. Chem. A 3 16272-8 DOI: 10.1039/C5TA02450A
|
| [45] |
Chen T, Liu W, Gao H, Zhuo Y, Hu H, Chen A, Zhang J, Yan J, Liu K 2018 A P2-type Na0.44Mn0.6Ni0.3Cu0.1O2 cathode material with high energy density for sodium-ion batteries J. Mater. Chem. A 6 12582-8 DOI: 10.1039/C8TA04791J
|
| [46] |
Wang L, Sun Y-G, Hu -L-L, Piao J-Y, Guo J, Manthiram A, Ma J, Cao A-M 2017 Copper-substituted Na0.67Ni0.3-xCuxMn0.7O2 cathode materials for sodium-ion batteries with suppressed P2-O2 phase transition J. Mater. Chem. A 5 8752-61 DOI: 10.1039/C7TA00880E
|
| [47] |
Yuan D, He W, Pei F, Wu F, Wu Y, Qian J, Cao Y, Ai X, Yang H 2013 Synthesis and electrochemical behaviors of layered Na0.67[Mn0.65Co0.2Ni0.15]O2 microflakes as a stable cathode material for sodium-ion batteries J. Mater. Chem. A 1 3895-9 DOI: 10.1039/c3ta01430d
|
| [48] |
Yuan D, Hu X, Qian J, Pei F, Wu F, Mao R, Ai X, Yang H, Cao Y 2014 P2-type Na0.67Mn0.65Fe0.2Ni0.15O2 cathode material with high-capacity for sodium-ion battery Electrochim. Acta 116 300-5 DOI: 10.1016/j.electacta.2013.10.211
|
| [49] |
Buchholz D, Moretti A, Kloepsch R, Nowak S, Siozios V, Winter M, Passerini S 2013 Toward Na-ion batteriessynthesis and characterization of a novel high capacity Na ion intercalation material Chem. Mater. 25 142-8 DOI: 10.1021/cm3029615
|
| [50] |
Yoshida J, Guerin E, Arnault M, Constantin C, Mortemard de Boisse B, Carlier D, Guignard M, Delmas C 2014 New P2Na0.70Mn0.60Ni0.30Co0.10O2 layered oxide as electrode material for Na-ion batteries J. Electrochem. Soc. 161 A1987-91 DOI: 10.1149/2.0121414jes
|
| [51] |
Luo C, Langrock A, Fan X, Liang Y, Wang C 2017 P2-type transition metal oxides for high performance Na-ion battery cathodes J. Mater. Chem. A 5 18214-20 DOI: 10.1039/C7TA04515H
|
| [52] |
Lu Z, Dahn J R 2001 In Situ x-ray diffraction study of P2-Na2/3[Ni1/3Mn2/3]O2 J. Electrochem. Soc. 148 A1225 DOI: 10.1149/1.1407247
|
| [53] |
Xu S, et al 2018 Suppressing the voltage decay of low-cost P2-type iron-based cathode materials for sodium-ion batteries J. Mater. Chem. A 6 20795-803 DOI: 10.1039/C8TA07933A
|
| [54] |
Talaie E, Duffort V, Smith H L, Fultz B, Nazar L F 2015 Structure of the high voltage phase of layered P2-Na2/3-z[Mn1/2Fe1/2]O2 and the positive effect of Ni substitution on its stability Energy Environ. Sci. 8 2512-23 DOI: 10.1039/C5EE01365H
|
| [55] |
Wang P-F, You Y, Yin Y-X, Wang Y-S, Wan L-J, Gu L, Guo Y-G 2016 Suppressing the P2-O2 phase transition of Na0.67Mn0.67Ni0.33O2 by magnesium substitution for improved sodium-ion batteries Angew. Chem., Int. Ed. Engl. 55 7445-9 DOI: 10.1002/anie.201602202
|
| [56] |
Hwang J-Y, Kim J, Yu T-Y, Sun Y-K 2019 A new P2-type layered oxide cathode with extremely high energy density for sodium-ion batteries Adv. Energy Mater. 9 1803346 DOI: 10.1002/aenm.201803346
|
| [57] |
Somerville J W, et al 2019 Nature of the Z-phase in layered Na-ion battery cathodes Energy Environ. Sci. 12 2223-32 DOI: 10.1039/C8EE02991A
|
| [58] |
Zhang L, Tsolakidou C, Mariyappan S, Tarascon J-M, Trabesinger S 2021 Unraveling gas evolution in sodium batteries by online electrochemical mass spectrometry Energy Storage Mater. 42 12-21 DOI: 10.1016/j.ensm.2021.07.005
|
| [59] |
Hatsukade T, Schiele A, Hartmann P, Brezesinski T, Janek J 2018 Origin of carbon dioxide evolved during cycling of nickel-rich layered NCM cathodes ACS Appl. Mater. Interfaces 10 38892-9 DOI: 10.1021/acsami.8b13158
|
| [60] |
Papp J K, Li N, Kaufman L A, Naylor A J, Younesi R, Tong W, McCloskey B D 2021 A comparison of high voltage outgassing of LiCoO2, LiNiO2, and Li2MnO3 layered Li-ion cathode materials Electrochim. Acta 368 137505 DOI: 10.1016/j.electacta.2020.137505
|
| [61] |
Strauss F, Payandeh S, Kondrakov A, Brezesinski T 2022 On the role of surface carbonate species in determining the cycling performance of all-solid-state batteries Mater. Futures 1 023501 DOI: 10.1088/2752-5724/ac5b7d
|
| [62] |
Dreyer S L, Kondrakov A, Janek J, Brezesinski T 2022 In situ analysis of gas evolution in liquid- and solid-electrolyte-based batteries with current and next-generation cathode materials J. Mater. Res. DOI: 10.1557/s43578-022-00586-2
|
| [63] |
Jung R, Metzger M, Maglia F, Stinner C, Gasteiger H A 2017 Oxygen release and its effect on the cycling stability of LiNixMnyCozO2 (NMC) cathode materials for Li-ion batteries J. Electrochem. Soc. 164 A1361-77 DOI: 10.1149/2.0021707jes
|
| [64] |
Wandt J, Freiberg A T S, Ogrodnik A, Gasteiger H A 2018 Singlet oxygen evolution from layered transition metal oxide cathode materials and its implications for lithium-ion batteries Mater. Today 21 825-33 DOI: 10.1016/j.mattod.2018.03.037
|
| [65] |
Metzger M, Strehle B, Solchenbach S, Gasteiger H A 2016 Origin of H2 evolution in LIBs: H2O reduction vs. Electrolyte oxidation J. Electrochem. Soc. 163 A798-809 DOI: 10.1149/2.1151605jes
|
| [66] |
Luo K, et al 2016 Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen Nat. Chem. 8 684-91 DOI: 10.1038/nchem.2471
|
| [67] |
House R A, et al 2019 What triggers oxygen loss in oxygen redox cathode materials? Chem. Mater. 31 3293-300 DOI: 10.1021/acs.chemmater.9b00227
|
| [68] |
Xu H, Guo S, Zhou H 2019 Review on anionic redox in sodium-ion batteries J. Mater. Chem. A 7 23662-78 DOI: 10.1039/C9TA06389G
|
| [69] |
Park J-H, I-h K, Lee J, Park S, Kim D, Yu S-H, Sung Y-E 2021 Anionic redox reactions in cathodes for sodium-ion batteries ChemElectroChem 8 625-43 DOI: 10.1002/celc.202001383
|
| [70] |
Solchenbach S, Hong G, Freiberg A T S, Jung R, Gasteiger H A 2018 Electrolyte and SEI decomposition reactions of transition metal ions investigated by on-line electrochemical mass spectrometry J. Electrochem. Soc. 165 A3304-12 DOI: 10.1149/2.0511814jes
|
| [71] |
Schwenke K U, Solchenbach S, Demeaux J, Lucht B L, Gasteiger H A 2019 The impact of CO2 evolved from VC and FEC during formation of graphite anodes in lithium-ion batteries J. Electrochem. Soc. 166 A2035-47 DOI: 10.1149/2.0821910jes
|
| [72] |
Wang B, Zhang F, Zhou X, Wang P, Wang J, Ding H, Dong H, Liang W, Zhang N, Li S 2021 Which of the nickel-rich NCM and NCA is structurally superior as a cathode material for lithium-ion batteries? J. Mater. Chem. A 9 13540-51 DOI: 10.1039/D1TA01128F
|
| [73] |
Sathiya M, et al 2015 Origin of voltage decay in high-capacity layered oxide electrodes Nat. Mater. 14 230-8 DOI: 10.1038/nmat4137
|
| [74] |
Li W, Liu X, Celio H, Smith P, Dolocan A, Chi M, Manthiram A 2018 Mn versus Al in layered oxide cathodes in lithium-ion batteries: a comprehensive evaluation on long-term cyclability Adv. Energy Mater. 8 1703154 DOI: 10.1002/aenm.201703154
|
| [1] | Meng Liu, Shoucong Ning, Dongdong Xiao, Yongzheng Zhang, Jiuhui Han, Chao Li, Anmin Nie, Xiang Zhang, Ao Zhang, Xiangrui Feng, Yujin Zhang, Weihua Wang, Zhen Lu, Haiyang Bai. Amorphous/crystalline heterostructured nanoporous high-entropy metallic glasses for efficient water splitting[J]. Materials Futures, 2025, 4(2): 025303. DOI: 10.1088/2752-5724/add415 |
| [2] | Xiaoming Lin, Jia Lin, Xiaomeng Lu, Xiaohong Tan, Hao Li, Wanxin Mai, Yuhong Luo, Yongbo Wu, Shuangqiang Chen, Chao Yang, Yong Wang. Vacancy-engineered LiMn2O4 embedded in dual-heteroatom-doped carbon via metal-organic framework-mediated synthesis towards longevous lithium ion battery[J]. Materials Futures, 2025, 4(2): 025101. DOI: 10.1088/2752-5724/ad9e08 |
| [3] | Lijin Dai, Changhui Song, Houxiong Fu, Hongyi Chen, Zhongwei Yan, Zibin Liu, Renyao Li, Anming Wang, Yongqiang Yang, Jia-Kuo Yu. Recrystallization induced by heat treatment regulates the anisotropic behavior of CoCrMo alloys fabricated by laser powder bed fusion[J]. Materials Futures, 2025, 4(2): 025001. DOI: 10.1088/2752-5724/adb50a |
| [4] | Q Xu, J Eckert, D Şopu. Improved irradiation resistance of high entropy nanolaminates through interface engineering[J]. Materials Futures, 2025, 4(1): 015301. DOI: 10.1088/2752-5724/ada8c5 |
| [5] | Jing Lin, Mareen Schaller, Ruizhuo Zhang, Volodymyr Baran, Hao Liu, Ziming Ding, Sylvio Indris, Aleksandr Kondrakov, Torsten Brezesinski, and Florian Strauss. High-Entropy Argyrodite Glass-Ceramic Electrolytes for All-Solid-State Batteries[J]. Materials Futures. DOI: 10.1088/2752-5724/adde76 |
| [6] | Zhong Yang, Xianglin Xiang, Jian Yang, Zong-Yan Zhao. High-entropy oxides as energy materials: from complexity to rational design[J]. Materials Futures, 2024, 3(4): 042103. DOI: 10.1088/2752-5724/ad8463 |
| [7] | Siyu An, Leonhard Karger, Sören L Dreyer, Yang Hu, Eduardo Barbosa, Ruizhuo Zhang, Jing Lin, Maximilian Fichtner, Aleksandr Kondrakov, Jürgen Janek, Torsten Brezesinski. Improving cycling performance of the NaNiO2 cathode in sodium-ion batteries by titanium substitution[J]. Materials Futures, 2024, 3(3): 035103. DOI: 10.1088/2752-5724/ad5faa |
| [8] | Huanbin Zheng, Jun Zeng, Xuanhong Wan, Xin Song, Chenxi Peng, Jiarui Wang, Luyi Sun, Hui Wang, Min Zhu, Jun Liu. ICE optimization strategies of hard carbon anode for sodium-ion batteries: from the perspective of material synthesis[J]. Materials Futures, 2024, 3(3): 032102. DOI: 10.1088/2752-5724/ad5d7f |
| [9] | Jijian Xu. High-entropy electrolytes in boosting battery performance[J]. Materials Futures, 2023, 2(4): 047501. DOI: 10.1088/2752-5724/ace8ab |
| [10] | Jiahao Zhang, Chao Ye, Yao Liao, Caihong Sun, Youlian Zeng, Jing Xiao, Zhi Chen, Wei Liu, Xiukang Yang, Ping Gao. Thiophene-functionalized porphyrin complexes as high performance electrodes for sodium ion batteries[J]. Materials Futures, 2023, 2(3): 035101. DOI: 10.1088/2752-5724/acdd86 |
| 1. | Yang, L., Geng, H., Guan, Z. et al. The role of La and Nd in enhancing CMAS corrosion resistance of high-entropy (La, Nd, Tm, Yb, Lu)2Zr2O7 thermal barrier coating materials. Journal of the European Ceramic Society, 2025, 45(12): 117466. DOI:10.1016/j.jeurceramsoc.2025.117466 | |
| 2. | Ma, H., Yin, B., Ye, T. et al. Constructing lithiophilic coating layer on the natural graphite for high-rate lithium-ion batteries. Electrochimica Acta, 2025. DOI:10.1016/j.electacta.2025.146275 | |
| 3. | Yang, L., Xie, F., Geng, H. et al. A novel (Ho0.2Er0.2Tm0.2Yb0.2Lu0.2)2Zr2O7 high-entropy ceramic with excellent CMAS corrosion resistance for thermal barrier coatings. Corrosion Science, 2025. DOI:10.1016/j.corsci.2025.112904 | |
| 4. | Ma, Y., Du, H., Zheng, S. et al. High-Entropy Approach vs. Traditional Doping Strategy for Layered Oxide Cathodes in Alkali-Metal-Ion Batteries: A Comparative Study. Energy Storage Materials, 2025. DOI:10.1016/j.ensm.2025.104295 | |
| 5. | Zhou, Y., Zhao, M., Bai, K. et al. Research on the construction of high-stability O3-type sodium-ion battery cathode materials via B-Co doping based on solid solutions. Chemical Engineering Journal, 2025. DOI:10.1016/j.cej.2025.161943 | |
| 6. | Ali, M., Saleem, M., Sattar, T. et al. High-entropy battery materials: Revolutionizing energy storage with structural complexity and entropy-driven stabilization. Materials Science and Engineering R: Reports, 2025. DOI:10.1016/j.mser.2024.100921 | |
| 7. | Zhang, J., Wu, T., Zhao, X. et al. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium–ion Batteries | [钠离子电池正极材料循环稳定性提升策略及产业化进程]. Wuji Cailiao Xuebao/Journal of Inorganic Materials, 2025, 40(4): 348-362. DOI:10.15541/jim20240368 | |
| 8. | Wang, Y., Geng, H., Yang, L. et al. Preparation, Microstructure, and Thermophysical Properties of a Novel (La, Nd, Tm, Yb, Lu)2Zr2O7 High-Entropy Ceramic for Thermal Barrier Coatings. Advanced Engineering Materials, 2025, 27(7): 2402593. DOI:10.1002/adem.202402593 | |
| 9. | Wang, L., Wang, L., Wang, H. et al. Progress and Perspective of High-Entropy Strategy Applied in Layered Transition Metal Oxide Cathode Materials for High-Energy and Long Cycle Life Sodium-Ion Batteries. Advanced Functional Materials, 2025, 35(11): 2417258. DOI:10.1002/adfm.202417258 | |
| 10. | Liu, S., Liu, F., Zhao, S. et al. A High-Entropy Engineering on Sustainable Anionic Redox Mn-Based Cathode with Retardant Stress for High-Rate Sodium-Ion Batteries. Angewandte Chemie - International Edition, 2025, 64(10): e202421089. DOI:10.1002/anie.202421089 | |
| 11. | Zhang, W., Han, P., Liu, Y. et al. Improvement strategies and research progress of silicon/graphite composites in lithium-ion batteries. FlatChem, 2025. DOI:10.1016/j.flatc.2025.100833 | |
| 12. | Jeong, S.H., Kim, I.-K., Eom, S. et al. Engineering the local chemistry through fe substitution in layered P2-Na0.7Ni0.2Co0.2Mn0.6O2 for high-performance Sodium-Ion batteries. Energy Storage Materials, 2025. DOI:10.1016/j.ensm.2025.104041 | |
| 13. | Li, H., Sun, X., Huang, H. The concept of high entropy for rechargeable batteries. Progress in Materials Science, 2025. DOI:10.1016/j.pmatsci.2024.101382 | |
| 14. | Cao, M., Cui, M., Gong, Y. et al. A novel high-entropy layered cathode with a robust structure and fast dynamics at high rates for Na-ion batteries. Sustainable Energy and Fuels, 2025, 9(4): 1062-1072. DOI:10.1039/d4se01225a | |
| 15. | Zheng, Y., Meng, Y., Hu, X. et al. Synthesis-Structure-Property of High-Entropy Layered Oxide Cathode for Li/Na/K-Ion Batteries. Advanced Materials, 2025, 37(1): 2413202. DOI:10.1002/adma.202413202 | |
| 16. | Duan, L., Zhang, Y., Tang, H. et al. Recent Advances in High-Entropy Layered Oxide Cathode Materials for Alkali Metal-Ion Batteries. Advanced Materials, 2025, 37(1): 2411426. DOI:10.1002/adma.202411426 | |
| 17. | Tsydypylov, D.Z., Slobodyuk, A.B., Kirsanova, M.A. et al. The effect of sodium content on sodium diffusion in NaxTi0.2Mn0.2Fe0.2Co0.2Ni0.2O2 high-entropy layered oxide. Journal of Solid State Electrochemistry, 2025. DOI:10.1007/s10008-024-06184-y | |
| 18. | He, L., Feng, T., Wu, Q. et al. High-voltage stabilized high-entropy oxyfluoride cathode for high-rate sodium-ion batteries. Rare Metals, 2025. DOI:10.1007/s12598-025-03318-7 | |
| 19. | Zulkifli, Sambandam, B., Fahri, A.N., Lee, S. et al. Sodium-Rich, Co-Ni-Free P2-Layered Manganese Oxide Cathodes for Sodium-Ion Batteries. Small, 2024, 20(51): 2404280. DOI:10.1002/smll.202404280 | |
| 20. | Yang, Z., Xiang, X., Yang, J. et al. High-entropy oxides as energy materials: from complexity to rational design. Materials Futures, 2024, 3(4): 042103. DOI:10.1088/2752-5724/ad8463 | |
| 21. | Pfeiffer, L.F., Dillenz, M., Burgard, N. et al. From structure to electrochemistry: the influence of transition metal ordering on Na+/vacancy orderings in P2-type NaxMO2 cathode materials for sodium-ion batteries. Journal of Materials Chemistry A, 2024, 13(1): 540-560. DOI:10.1039/d4ta04786a | |
| 22. | Doan, T.P., To Van, N., Nguyen Van, K. et al. Development of Sodium-Lithium-Manganese-Cobalt Oxide with B Doping or B/F Dual Doping as Cathode Electrode Materials for Sodium-Ion Batteries. ACS Omega, 2024, 9(47): 46916-46928. DOI:10.1021/acsomega.4c06248 | |
| 23. | Zhou, Z., Ma, Y., Brezesinski, T. et al. Improving upon rechargeable battery technologies: on the role of high-entropy effects. Energy and Environmental Science, 2024, 18(1): 19-52. DOI:10.1039/d4ee03708a | |
| 24. | Maresca, G., Ottaviani, M., Ryan, K.M. et al. Improved Compatibility of α-NaMnO2 Cathodes at the Interface with Ionic Liquid Electrolytes. ChemSusChem, 2024, 17(21): e202400514. DOI:10.1002/cssc.202400514 | |
| 25. | Chen, Y., Li, P., Huang, M. et al. Elucidating the role of embedding dispersed cobalt sites in nitrogen-doped carbon frameworks in Si-based anodes for stable and superior storage. Journal of Energy Chemistry, 2024. DOI:10.1016/j.jechem.2024.06.022 | |
| 26. | Zhou, B., Wong, D., Fu, Z. et al. K-Doping Suppresses Oxygen Redox in P2-Na0.67Ni0.11Cu0.22Mn0.67O2 Cathode Materials for Sodium-Ion Batteries. Small, 2024, 20(43): 2402991. DOI:10.1002/smll.202402991 | |
| 27. | Dong, Y., Zhou, Z., Ma, Y. et al. Layered-Structured Sodium-Ion Cathode Materials: Advancements through High-Entropy Approaches. ACS Energy Letters, 2024, 9(10): 5096-5119. DOI:10.1021/acsenergylett.4c02223 | |
| 28. | Xu, X., Liu, Q., Cao, S. et al. Facilitating superior electrochemical efficacy and ultra rapid kinetic in Co/Ni-free layered oxides for sodium-ion batteries with phosphate-growing layer fast ion conductors. Chemical Engineering Journal, 2024. DOI:10.1016/j.cej.2024.155685 | |
| 29. | El Moutchou, S., Sabi, N., Oueldna, N. et al. High-entropy cathode materials for sodium-ion batteries: Correlating synthesis, crystal structure and electrochemical properties. Journal of Energy Storage, 2024. DOI:10.1016/j.est.2024.113078 | |
| 30. | He, Y., Dreyer, S.L., Akçay, T. et al. Leveraging Entropy and Crystal Structure Engineering in Prussian Blue Analogue Cathodes for Advancing Sodium-Ion Batteries. ACS Nano, 2024, 18(35): 24441-24457. DOI:10.1021/acsnano.4c07528 | |
| 31. | Hou, P., Gong, M., Dong, M. et al. The emerging high-entropy cathode materials for advanced Na-ion batteries: advances and perspectives. Energy Storage Materials, 2024. DOI:10.1016/j.ensm.2024.103750 | |
| 32. | An, S., Karger, L., Dreyer, S.L. et al. Improving cycling performance of the NaNiO2 cathode in sodium-ion batteries by titanium substitution. Materials Futures, 2024, 3(3): 035103. DOI:10.1088/2752-5724/ad5faa | |
| 33. | Huang, Y., Zeng, W., Li, K. et al. Na-deficient P2-type layered oxide cathodes for practical sodium-ion batteries. Microstructures, 2024, 4(3): 2024027. DOI:10.20517/microstructures.2023.102 | |
| 34. | Huang, L., Zhu, J., Liu, J.-X. et al. Emerging high-entropy strategy: A booster to the development of cathode materials for power batteries. Journal of Advanced Ceramics, 2024, 13(8): 1093-1118. DOI:10.26599/JAC.2024.9220913 | |
| 35. | Ran, B., Li, H., Cheng, R. et al. High-Entropy Oxides for Rechargeable Batteries. Advanced Science, 2024, 11(25): 2401034. DOI:10.1002/advs.202401034 | |
| 36. | Li, N., Yin, W., Wang, B. et al. Lowering Sodium-Storage Lattice Strains of Layered Oxide Cathodes by Pushing Charge Transfer on Anions. Energy and Environmental Materials, 2024, 7(4): e12671. DOI:10.1002/eem2.12671 | |
| 37. | Li, X., Wang, Y., Lu, J. et al. Constructing static two-electron lithium-bromide battery. Science Advances, 2024, 10(24): eadl0587. DOI:10.1126/sciadv.adl0587 | |
| 38. | Gao, H., Li, J., Zhang, F. et al. Revealing the Potential and Challenges of High-Entropy Layered Cathodes for Sodium-Based Energy Storage. Advanced Energy Materials, 2024, 14(20): 2304529. DOI:10.1002/aenm.202304529 | |
| 39. | Bao, C., Chu, P., Xu, C. et al. More disorder is better: Cutting-edge progress of high entropy materials in electrochemical energy storage applications. Energy Storage Materials, 2024. DOI:10.1016/j.ensm.2024.103408 | |
| 40. | Gorbunov, M.V., Mikhailova, D. Structural Behaviour and Charge-Compensation Mechanism in Li2Fe1−xCoxSeO Solid Solutions during Reversible Delithiation. Processes, 2024, 12(4): 756. DOI:10.3390/pr12040756 | |
| 41. | Schweidler, S., Botros, M., Strauss, F. et al. High-entropy materials for energy and electronic applications. Nature Reviews Materials, 2024, 9(4): 266-281. DOI:10.1038/s41578-024-00654-5 | |
| 42. | Dreyer, S.L., Maddar, F.M., Kondrakov, A. et al. Elucidating Gas Evolution of Prussian White Cathodes for Sodium-ion Battery Application: The Effect of Electrolyte and Moisture. Batteries and Supercaps, 2024, 7(4): e202300595. DOI:10.1002/batt.202300595 | |
| 43. | Strauss, F., Botros, M., Breitung, B. et al. High-entropy and compositionally complex battery materials. Journal of Applied Physics, 2024, 135(12): 120901. DOI:10.1063/5.0200031 | |
| 44. | Schweidler, S., Brezesinski, T., Breitung, B. Entropy-assisted epitaxial coating. Nature Energy, 2024, 9(3): 240-241. DOI:10.1038/s41560-024-01468-z | |
| 45. | Garcia, N.G., Gonçalves, J.M., Real, C. et al. Medium- and high-entropy materials as positive electrodes for sodium-ion batteries: Quo Vadis?. Energy Storage Materials, 2024. DOI:10.1016/j.ensm.2024.103213 | |
| 46. | Mathiyalagan, K., Shin, D., Lee, Y.-C. Difficulties, strategies, and recent research and development of layered sodium transition metal oxide cathode materials for high-energy sodium-ion batteries. Journal of Energy Chemistry, 2024. DOI:10.1016/j.jechem.2023.10.023 | |
| 47. | He, Y., Dreyer, S.L., Ting, Y.-Y. et al. Entropy-Mediated Stable Structural Evolution of Prussian White Cathodes for Long-Life Na-Ion Batteries. Angewandte Chemie - International Edition, 2024, 63(7): e202315371. DOI:10.1002/anie.202315371 | |
| 48. | Fan, L., Shu, G., Liu, Y. et al. Cu-substituted nickel hexacyanoferrate with tunable reaction potentials for superior ammonium ion storage. Journal of Materials Science and Technology, 2024. DOI:10.1016/j.jmst.2023.06.010 | |
| 49. | Spiridigliozzi, L.. Order from Chaos: Theoretical Principles and Practical Aspects of the New Class ofHigh-Entropy Materials. Order from Chaos: Theoretical Principles and Practical Aspects of the New Class of High-Entropy Materials, 2024. DOI:10.1201/9781003346807 | |
| 50. | Kuzmin, A.. X-ray absorption spectroscopy in high-entropy material research. High-Entropy Alloys: Design, Manufacturing, and Emerging Applications, 2024. DOI:10.1016/B978-0-443-22142-2.00006-5 | |
| 51. | Gauckler, C., Kucinskis, G., Pfeiffer, L.F. et al. MgO coated P2-Na0.67Mn0.75Ni0.25O2 layered oxide cathode for Na-Ion batteries. Journal of Power Sources Advances, 2024. DOI:10.1016/j.powera.2024.100135 | |
| 52. | Joshi, A., Chakrabarty, S., Akella, S.H. et al. High-Entropy Co-Free O3-Type Layered Oxyfluoride: A Promising Air-Stable Cathode for Sodium-Ion Batteries. Advanced Materials, 2023, 35(51): 2304440. DOI:10.1002/adma.202304440 | |
| 53. | Nowak, M., Walczak, K., Milewska, A. et al. Electrochemical performance of different high-entropy cathode materials for Na-ion batteries. Journal of Alloys and Compounds, 2023. DOI:10.1016/j.jallcom.2023.172316 | |
| 54. | Britala, L., Marinaro, M., Kucinskis, G. A review of the degradation mechanisms of NCM cathodes and corresponding mitigation strategies. Journal of Energy Storage, 2023. DOI:10.1016/j.est.2023.108875 | |
| 55. | Singh, A.N., Islam, M., Meena, A. et al. Unleashing the Potential of Sodium-Ion Batteries: Current State and Future Directions for Sustainable Energy Storage. Advanced Functional Materials, 2023, 33(46): 2304617. DOI:10.1002/adfm.202304617 | |
| 56. | Xu, W., Dang, R., Zhou, L. et al. Conversion of Surface Residual Alkali to Solid Electrolyte to Enable Na-Ion Full Cells with Robust Interfaces. Advanced Materials, 2023, 35(42): 2301314. DOI:10.1002/adma.202301314 | |
| 57. | Zheng, Y., Kong, X., He, L. et al. Constructing bimetallic heterostructure as anodes for sodium storage with superior stability and high capacity. Journal of Power Sources, 2023. DOI:10.1016/j.jpowsour.2023.233371 | |
| 58. | Zhang, S., Li, X., Su, Y. et al. Four-In-One Strategy to Boost the Performance of Nax[Ni, Mn]O2. Advanced Functional Materials, 2023, 33(36): 2301568. DOI:10.1002/adfm.202301568 | |
| 59. | Zhang, J., Ye, C., Liao, Y. et al. Thiophene-functionalized porphyrin complexes as high performance electrodes for sodium ion batteries. Materials Futures, 2023, 2(3): 035101. DOI:10.1088/2752-5724/acdd86 | |
| 60. | Thangavel Senthilkumar, S., Marcilla, R., Kim, Y. et al. Rechargeable Na-MnO2 battery with modified cell chemistry. Journal of Energy Chemistry, 2023. DOI:10.1016/j.jechem.2023.05.044 | |
| 61. | Mu, J., Cai, T., Dong, W. et al. Biphasic high-entropy layered oxide as a stable and high-rate cathode for sodium-ion batteries. Chemical Engineering Journal, 2023. DOI:10.1016/j.cej.2023.144403 | |
| 62. | Zhang, Y.-H., Wang, J.-L., Yan, H.-Y. et al. Short rod-like NiCoSe2 binary-metal selenide nanomaterials of carbon-coated as high-performance anode for sodium-ion batteries. Ionics, 2023, 29(9): 3505-3515. DOI:10.1007/s11581-023-05076-x | |
| 63. | Karkera, G., Soans, M., Akbaş, A. et al. A Structurally Flexible Halide Solid Electrolyte with High Ionic Conductivity and Air Processability. Advanced Energy Materials, 2023, 13(30): 2300982. DOI:10.1002/aenm.202300982 | |
| 64. | Liu, Z., Wu, J., Zeng, J. et al. Co-Free Layered Oxide Cathode Material with Stable Anionic Redox Reaction for Sodium-Ion Batteries. Advanced Energy Materials, 2023, 13(29): 2301471. DOI:10.1002/aenm.202301471 | |
| 65. | Dreyer, S.L., Zhang, R., Wang, J. et al. The effect of configurational entropy on acoustic emission of P2-type layered oxide cathodes for sodium-ion batteries. JPhys Energy, 2023, 5(3): 035002. DOI:10.1088/2515-7655/acd41a | |
| 66. | Chen, J., Adit, G., Li, L. et al. Optimization Strategies Toward Functional Sodium-Ion Batteries. Energy and Environmental Materials, 2023, 6(4): e12633. DOI:10.1002/eem2.12633 | |
| 67. | Ko, D., Mhin, S. Effect of One Step Solid State Reaction Route on the Semiconductor Behavior of the Spinel (NI, Co, and Mn)O4 to Be Used as Temperature Sensor. Sensors, 2023, 23(12): 5380. DOI:10.3390/s23125380 | |
| 68. | Xu, M., Liu, Z., Li, Y. et al. Uniform SnSe nanoparticles on 3D graphene host enabling a dual-nucleation-site interface for dendrite-free sodium metal batteries. Energy Storage Materials, 2023. DOI:10.1016/j.ensm.2023.102848 | |
| 69. | Zeng, A., Jiao, J., Zhang, H. et al. Clarifying effects of in-plane cationic-ordering degree on anionic redox chemistry in Na-ion battery layered oxide cathodes. Materials Today Chemistry, 2023. DOI:10.1016/j.mtchem.2023.101532 | |
| 70. | Jiao, J., Song, H., Zhao, E. et al. Quantifying Effects of Ligand-Metal Bond Covalency on Oxygen-Redox Electrochemistry in Layered Oxide Cathodes. Inorganic Chemistry, 2023, 62(18): 7045-7052. DOI:10.1021/acs.inorgchem.3c00344 |