-
Abstract: The growing concern about scarcity and large-scale applications of lithium resources has attracted efforts to realize cost-effective phosphate-based cathode materials for next-generation Na-ion batteries (NIBs). In previous work, a series of materials (such as Na4Fe3(PO4)2(P2O7), Na3VCr(PO4)3, Na4VMn(PO4)3, Na3MnTi(PO4)3, Na3MnZr(PO4)3, etc) with 120 mAh g-1 specific capacity and high operating potential has been proposed. However, the mass ratio of the total transition metal in the above compounds is only 22 wt%, which means that one-electron transfer for each transition metal shows a limited capacity (the mass ratio of Fe is 35.4 wt% in LiFePO4). Therefore, a multi-electron transfer reaction is necessary to catch up to or go beyond the electrochemical performance of LiFePO4. This review summarizes the reported NASICON-type and other phosphate-based cathode materials. On the basis of the aforementioned experimental results, we pinpoint the multi-electron behavior of transition metals and shed light on designing rules for developing high-capacity cathodes in NIBs.
-
Keywords:
- NASICON /
- Na-ion batteries /
- cathode materials /
- multi-electron transfer reactions
-
1. Introduction
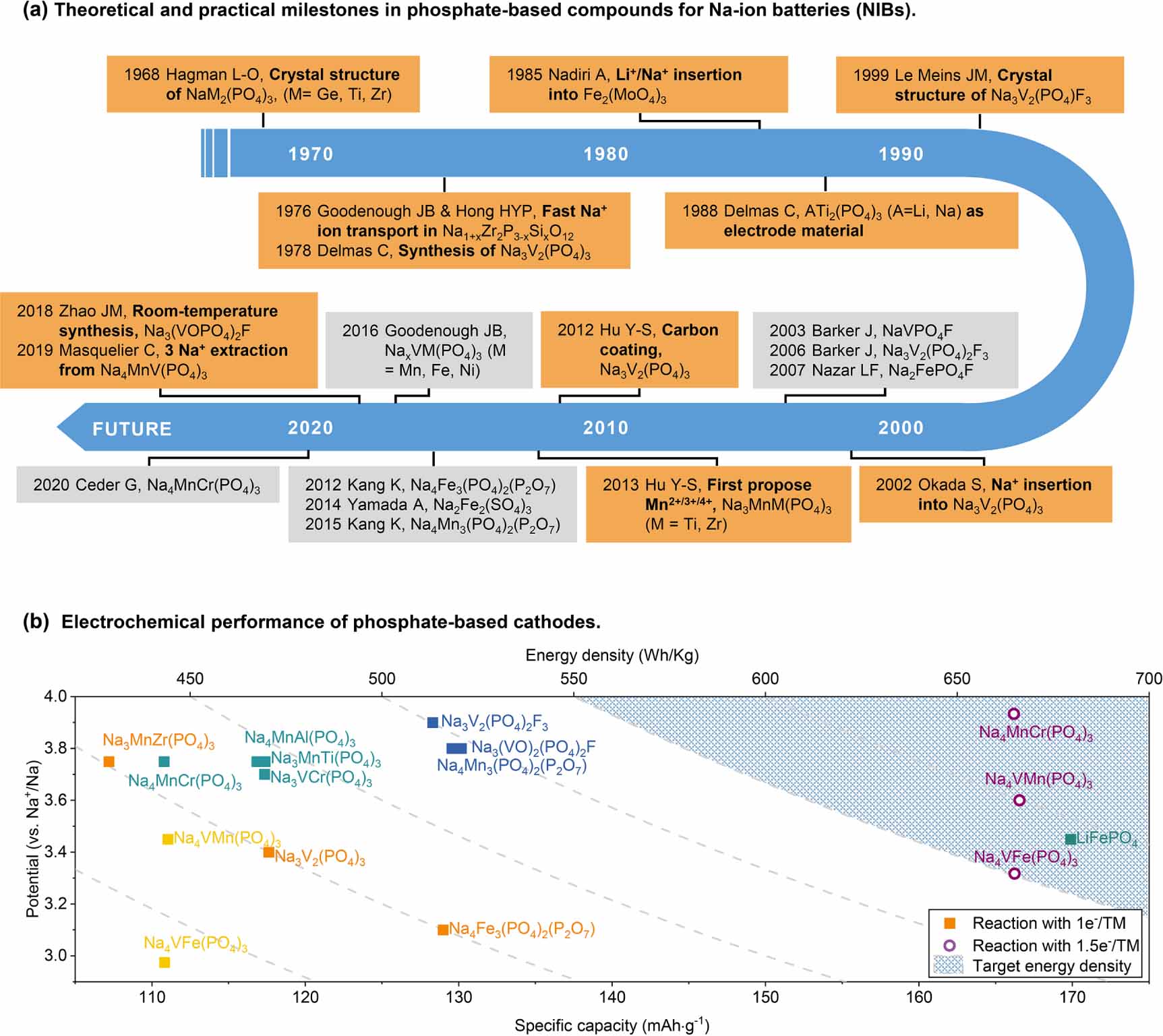
Large-scale applications based on Na-ion batteries (NIBs) are expected to integrate intermittent renewable energy sources because of the low cost, wide distribution, and abundant reserves of sodium resources [1-7], where the development of electrode materials is one of the most significant tasks for the improvement of NIBs. In terms of cathode materials, polyanion compounds have high safety and chemical/electrochemical stability [8], which could match the urgent requirement of grid energy storage devices. Among the phosphate-based cathodes, NASICON-type materials have attracted growing attention due to their high Na+ ion conductivity [9-12]. It took 30 years from identifying the crystal structure to realizing reversible charge/discharge behavior in the NIBs (figure 1(a)). As early as 1968, Hagman’s group [13] reported the NaMe2(PO4)3 (Me = Ge, Ti, Zr) structure. They mentioned that the crystal’s 3D framework is built up of the corner link of MeO6 octahedra and PO4 tetrahedra, and the oxygen atoms octahedrally surround the sodium atoms. In 1976, Goodenough and Hong et al [14, 15] found fast alkali-ion transport in a series of materials conforming to the chemical formula Na1 + xZr2P3-xSixO12(0 x 3). These compounds were named NASICON (sodium (Na) super (S) ion (I) conductor (CON)), benefiting from the three-dimensional diffusion tunnel. Subsequently, Nadiri et al [16] used Fe2(MoO4)3 for the positive electrode and AClO4 (1 M, A = Li/Na) in propylene carbonate as the electrolyte to fabricate half cells, revealing the intercalation behavior of alkali metal ions in the NASICON framework. In 1988, reversible electrochemical (de)intercalation was successfully realized in ATi2(PO4)3 for the first time [17]. However, much research focused on LIB material systems after the first commercial lithium-ion battery was issued in 1991. In 2002, Uebou et al reported electrochemical sodium insertion/extraction of the 3D framework of Na3V2(PO4)3 [18], which was synthesized by Delmas in 1978 [19]. However, the insufficient electrochemical data attracted limited attention to such materials until Hu’s group first proposed the carbon coating approach to significantly improve the cycling and rate performance [20].
![]() Figure 1. (a) Timeline of the development of phosphate-based cathode materials. (b) Potential and specific capacity of different cathode materials. Squares are one-electron reactions of each transition metal (named 1e-/TM); the circle symbols are 1.5e-/TM. NOTE: the reactions with 1.5e-/TM in Na4VMn(PO4)3 and Na4VFe(PO4)3 are irreversible.
Figure 1. (a) Timeline of the development of phosphate-based cathode materials. (b) Potential and specific capacity of different cathode materials. Squares are one-electron reactions of each transition metal (named 1e-/TM); the circle symbols are 1.5e-/TM. NOTE: the reactions with 1.5e-/TM in Na4VMn(PO4)3 and Na4VFe(PO4)3 are irreversible.Since then, Na3V2(PO4)3 has been regarded as a promising cathode candidate earning wide investigation, and several modification strategies have been explored to optimize its electrochemical performance [20-22]. However, vanadium’s high cost and low resource sustainability became one of the most serious bottlenecks contrary to the requirements of large-scale applications [23]. In 2013, Hu’s group proposed Mn2+/3+/4+ redox couples in NASICON-type cathodes, and kinds of Mn-rich compounds were designed (such as Na3MnTi(PO4)3 and Na3MnZr(PO4)3, etc) [24]. Subsequently, the reversible redox couples of Mn2+/3+/4+ in a NASICON-type cathode have been realized with a high operating potential in Na3MnTi(PO4)3 (3.6 V and 4.0 V) [25]. Furthermore, Fe-rich NASICON-type cathode materials (such as Na3Fe2(PO4)3) have attracted great interest due to their wide sources, low costs and abundant reserves on Earth. However, the limited thermodynamic equilibrium potential of Fe2+/3+ restricted the research of Fe-based NASICON-type cathodes. It is exciting that researchers found that P2O74- and F- can increase the redox potential based on Fe2+/3+ due to the strong electronegativity of such ions. As a result, a series of new structures has been discovered for phosphate-based mixed-polyanion cathodes [26-28]. However, the low transition metal mass fraction of the above compounds leads to a limited theoretical specific capacity, as shown in figure 1(b). Therefore, realizing the multi-electron transfer reaction is crucial for advanced next-generation NIBs.
In this review, we summarized redox couples with electrochemical activity in NASICON-type cathodes and other polyanionic compounds. Based on reported voltage profiles and previous accumulations on multi-electron transfer reactions, we pinpoint the reversibility of redox couples in NASICON-type cathodes closely related to the crystal structure. As a result, we demonstrate a cascade of guiding lines for enabling better designs of high-capacity polyanionic NIB cathodes.
2. Structure
NASICON-type cathode materials are increasingly attracting attention as phosphate-based compounds due to tunable transition metal sites and fast Na+ ion transport pathways. As early as 1976, a series of materials with the chemical formula Na1 + xZr2P3-xSixO12(0 x 3) were named NASICON (an acronym for sodium (Na) Super Ionic CONductor) [14]. Similarly, NASICON-type material structures usually refer to a family of solids with the chemical formula AMM’(PO4)3 [11]. Where the A’ site can be occupied by alkali ions (Li+, Na+, K+, Rb+, and Cs+), alkaline earth ions (Mg2+, Ca2+, Sr2+, and Ba2+), transition metals (Cu2+, Ag+, Pb2+, Cd2+, Mn2+, Co2+, Ni2+, Zn2+, Al3+, Ge4+, Zr4+, and Hf4+), and ion-molecules (H3O+ and NH4+), may also be vacancies. The M and M’ sites are divided by 3d (Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Zn), 4d (Y, Zr, Nb, Mo), 5d (Lu, Hf, Ta), and main-group (Al, Si, In, Ge, As, Sn, Sb) elements to balance the charge appropriately. Phosphorus can be partially or even entirely replaced by S, Si, As, W and Mo, while O can also be replaced by F and Cl. Furthermore, the crystal structure can be rhombohedral, monoclinic, triclinic, orthorhombic, garnet, SW-type, corundum, etc, with different elements. Notably, rhombohedral structures have been extensively reported due to their superior ion diffusion pathway. In this structure, MO6 and M’O6 octahedrons share all angles with XO4 tetrahedrons, and MO6 and M’O6 octahedrons are arranged linearly along the c-axis. The octahedron MO6 and M’O6 connect three tetrahedral XO4 units to form a basic unit called a lantern [29]. Each lantern is connected to six other lanterns, thereby constructing a stable 3D skeleton structure [30]. In this open 3D framework, interconnected channels provide a high-speed transmission pathway for the ions encapsulated in the A’ site. Intriguingly, its content is between 1 and 5 [31, 32]. In addition, it can be de-intercalated continuously without structural collapse.
3. Electrochemical performance of reported materials
The NASICON-type compounds are much favored by the open 3D diffusion channels and stable skeleton structure. Currently, research focuses on the following directions: optimizing strategies for Na3V2(PO4)3-based materials (e.g. carbon coating [20, 33-37], morphology control [22, 38], element doping [21, 39-45], etc), and exploring unknown material systems by replacing the M/M’ transition metal sites [25, 46-48]. To date, the NASICON-type cathode materials with various electrochemically active metals (V, Mn, Fe, Cr, Ti, etc) have been extensively explored. Meanwhile, phosphate-based mixed-polyanion compounds (such as Na3V2(PO4)2F3, Na4Fe3(PO4)2(P2O7), Na2FePO4F, etc) have also been proposed as cathodes in NIBs.
3.1 V-based NASICON cathodes
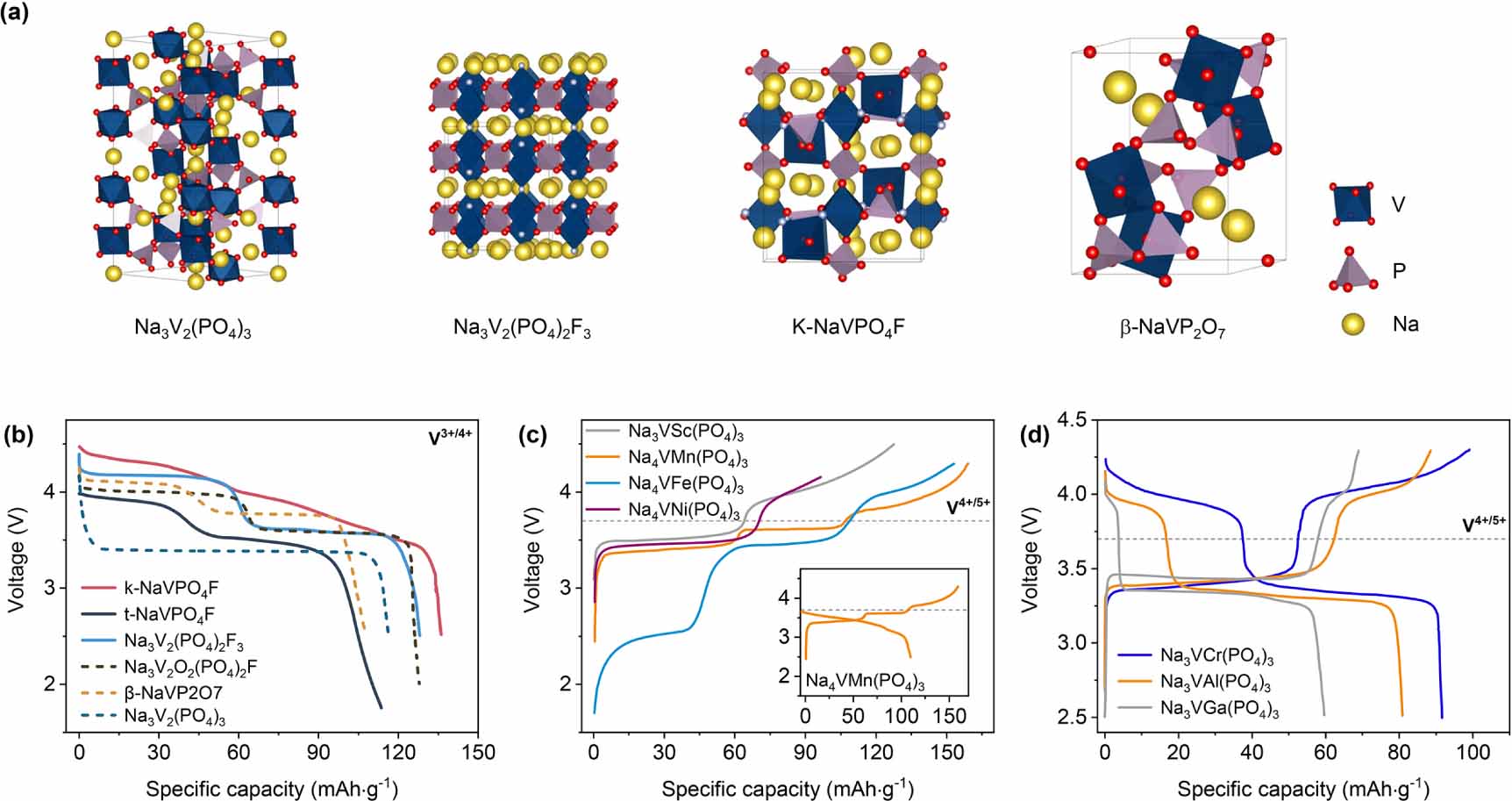
Vanadium compounds have attracted great attention for their excellent redox, electrochemical, catalytic, and magnetic properties [49-51]. Surprisingly, the vanadium atoms can adopt different oxidation states (from Ⅱ to V), coordination (from 6 to 4), and environments (octahedral to tetrahedral) in the reported vanadium phosphates. Abundant bonds lead to a wide variety of V-based polyanion compounds. Na3V2(PO4)3 can be indexed as the rhombohedral phase
Rˉ3C space group [29, 32, 52], and the oxidation state of V in Na3V2(PO4)3 is confirmed to be trivalent [31]. As a cathode material, it exhibits a theoretical specific capacity of 117.6 mAh g-1 with 3.4 V operating voltage (vs. Na+/Na). The incompletely occupied Na+ sites provide a fast ion diffusion channel, which enables the material to exhibit excellent rate capability [53]. Furthermore, Masquelier et al have made many contributions to elucidate the crystal structure and charge/discharge behavior of Na3V2(PO4)3 [32, 52].The transition metal substitution of the V element in Na3V2(PO4)3 has also been widely studied due to the high cost of V-based compounds. In 2016, Fe, Mn, and Ni were used to replace V to synthesize a series of materials of Na4VM(PO4)3 (M = Fe, Mn, Ni) [48]. Similar to LMFP, the operating potential of Na4VMn(PO4)3 can be significantly improved without capacity fading. Immediately, researchers focused on enhancing the electrochemical performance of Na4VMn(PO4)3 [54-60]. However, as shown in figure 2(c), the V4+/5+ redox couple is irreversible in Na4VMn(PO4)3. It should be noted that the multi-electron transfer reaction is one of the prerequisites for high capacity NASICON-type cathodes. Therefore, the failure mechanism and how to realize a reversible V4+/5+ redox couple are crucial. In 2020, Liu et al [61] revealed that the small ion radius of V5+ can migrate to Na_vacancy sites and block the sodium ion pathway in Na3VCr(PO4)3. Interestingly, a similar phenomenon was captured in Na3VSc(PO4)3, and a slightly reversible capacity at the 4.0 V platform was shown at -20 C [62]. Based on the above finding, we can speculate that the transition metal migration of V5+ is a common issue of the irreversible V4+/5+ redox couple. It should be noted that the replacing elements of Al3+, Cr3+, and Ga3+ make the V4+/5+ redox couple reaction reversible, and a platform located at 4.0 V (vs. Na+/Na) occurred in the discharge curve (figure 2(d)) [63-66]. This finding can be attributed to the small Al3+ and the eliminated Jahn-Teller effect of Mn3+, so the crystal structure can be stable. In addition, the modification of polyanion groups in the NASICON framework also can be considered [67]. However, the above conclusions are inferred based on the reported experimental results, and research on such topics is still limited [68]. Therefore, we must pay attention to such issues and draw a whole picture of failure mechanisms or optimization strategies.
![]() Figure 2. (a) Crystal structures and (b) voltage profiles of the typical V-based NASICON-type cathode materials, data from [69, 70, 79, 80]. (c) Charge curves of the V3+ to V5+ in which the V4+/5+ redox couple is irreversible, data from [54, 62]. (d) Voltage profiles of the reversible V4+/5+ redox couple reactions, data from [46].
Figure 2. (a) Crystal structures and (b) voltage profiles of the typical V-based NASICON-type cathode materials, data from [69, 70, 79, 80]. (c) Charge curves of the V3+ to V5+ in which the V4+/5+ redox couple is irreversible, data from [54, 62]. (d) Voltage profiles of the reversible V4+/5+ redox couple reactions, data from [46].Furthermore, benefitting from the stronger electronegativity of F-, the partial substitution of V-F for V-O can significantly improve the operating voltage of V-based cathode materials [50]. In recent years, fluorine-containing vanadium-based polyanion compounds such as NaVPO4F [69, 70], Na3V2(PO4)2F3 [71-74], and Na3V2O2x(PO4)2F3-2x [28, 75-77] have been reported as cathodes for NIBs (figures 2(a) and (b)). Although the above cathodes deliver a reversible specific capacity of 120 mAh g-1, the crystal structure will collapse when the V valence exceeds +4. In 2019, Yan et al [78] showed a detailed picture of the structural evolution of Na3V2(PO4)2F3 when more than 2.5 sodium ions were extracted. In addition, they revealed that the Na0V2(PO4)2F3 phase accommodates sodium in a disordered way and does not convert back to the initial structure. The aforementioned experimental results indicate that more than 1Na/TM can be extracted upon further charging, but the structural collapse occurs simultaneously. Therefore, this is the key problem of V-based high-capacity cathodes.
3.2 Mn-based NASICON cathodes
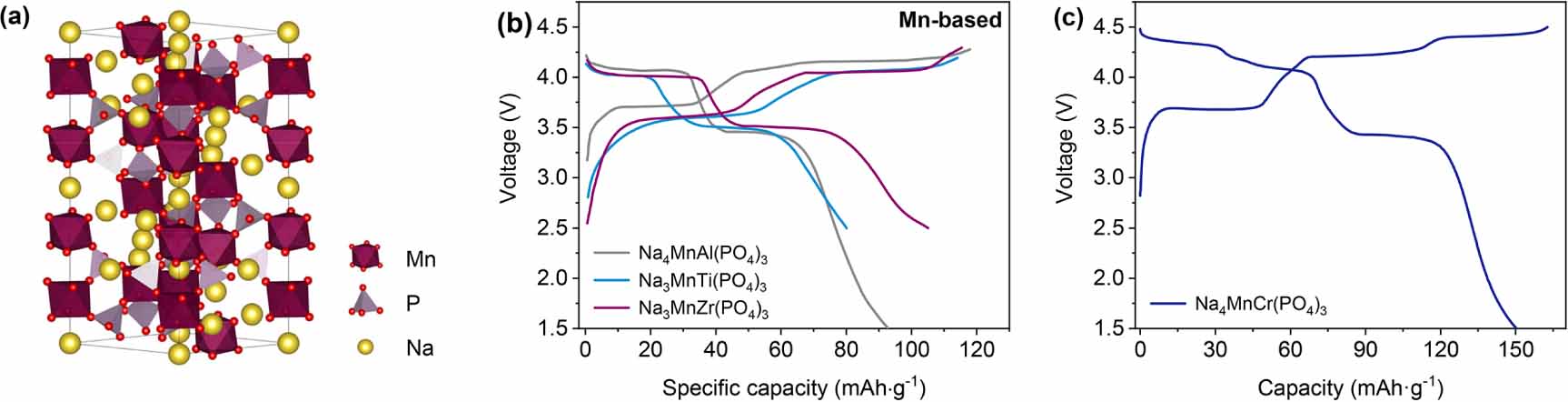
Manganese-based electrode materials are attractive due to their excellent stability, resource non-criticality, and high electrode potential [24, 81]. Currently, Mn-rich NASICON-type phosphates provide high electrode potentials and robust anionic redox-free frameworks, such as Na3MnTi(PO4)3 [25, 81-89], Na3MnZr(PO4)3 [47, 90], and Na4MnCr(PO4)3 [91-95] (figure 3(a)). In 2013, Pan et al [24] first proposed Na3MnTi(PO4)3 and Na3MnZr(PO4)3 as cathode materials for NIBs. Subsequently, Gao et al [25] successfully achieved a discharge capacity of 80 mAh g-1 in Na3MnTi(PO4)3. It should be noted that the Mn2+/3+ and Mn3+/4+ redox couples can be entirely activated within the voltage range of 2.5-4.2 V. The corresponding thermodynamic equilibrium potential is 3.6 V and 4.0 V, respectively. As shown in figure 3(b), the voltage profiles of Na4MnAl(PO4)3, Na3MnTi(PO4)3, and Na3MnZr(PO4)3 exhibit initial capacity fading and voltage hysteresis (Al3+ < Ti4+ < Zr4+). However, the significant capacity fading of Mn-rich NASICON-type cathodes arises in the initial cycle, and an outstanding cycling performance is displayed in the following cycles [86, 89]. This result means that the failure mechanism of Mn-based cathodes is different from that of V-based ones (V5+ migrates to Na_vacancy sites during charging). For the high-capacity cathode, Wang et al [93] found that Cr3+ can not only activate the Mn2+/4+ redox couple, but Cr3+/4+ is also electrochemically active in Na4MnCr(PO4)3. Therefore, Na4MnCr(PO4)3 exhibits a high reversible capacity of 150.3 mAh g-1, which is close to the 1.5e-/TM transfer reaction, as shown in figure 3(c). Unfortunately, the high operating voltage and poor stability limit its application, and more study is needed to optimize the electrochemical performance.
3.3 Fe-based NASICON cathodes
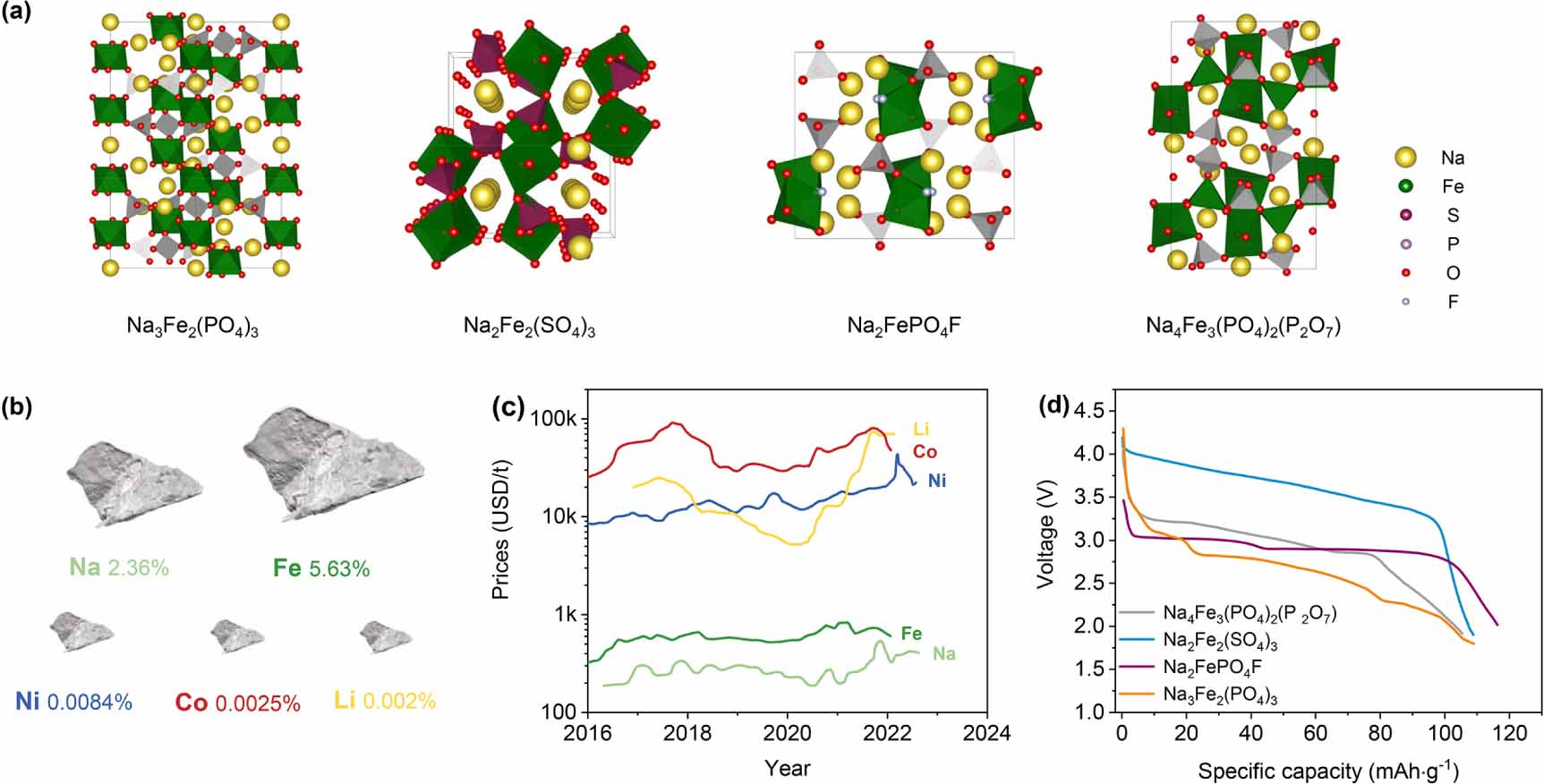
Fe-based phosphate compounds play a dominant role in NIB cathode research, which is encouraged by the success of LiFePO4 [97]. As shown in figures 4(b) and (c), the low cost and high resource abundance [23] of Fe/Na match the requirements of large-scale energy storage devices. However, the thermodynamically stable structure of NaFePO4 is an electrochemically inactive maricite phase [98]. Furthermore, the electrode potential of the Fe2+/3+ reversible redox couple is 2.4 V (vs. Na+/Na) in NASICON-type compounds [99-101]. The operating potential is too low for the cathode material. Subsequently, anion groups with stronger electronegativity are used to increase the thermodynamic equilibrium potential of the Na-Fe-P-O system (e.g. P2O74- [102-104], F- [105, 106], etc). In 2012, Barpanda et al [104] found that Na2FeP2O7 has a high theoretical specific capacity of 97 mAh g-1, with the thermodynamic equilibrium potential of the Fe2+/3+ redox couple raised to 3.0 V (vs. Na+/Na). However, the high molecular mass of P2O74- results in a limited capacity. Kim et al [26] developed a new mixed-polyanion cathode for NIBs, Na4Fe3(PO4)2(P2O7). Na4Fe3(PO4)2(P2O7) [107-109] shows a robust open framework with 3D Na+ ion diffusion paths. It is a new striking Fe-based polyanion material due to its high theoretical capacity (129 mAh g-1), with an average discharge potential of 3.1 V (vs. Na+/Na). In addition, Na2Fe2(SO4)3 [27, 110], Na3Fe2(PO4)(P2O7) [98, 105, 106], and Na2FePO4F [98, 105, 106] have also been considered candidates for advanced commercial NIB cathode materials (figure 4(d)).
![]() Figure 4. (a) Crystal structures of the typical Fe-based NASICON-type cathode materials. (b) Abundance in Earth’s crust [111] and (c) prices [112] of elements. (d) Potential vs. specific capacity plots for Na4Fe3(PO4)2(P2O7) [113], Na2Fe2(SO4)3 [108], Na2FePO4F [105], and Na3Fe2(PO4)3 [101] normalized to the theoretical specific capacity per one-electron Fe2+ Fe3+ transition from experimental data.
Figure 4. (a) Crystal structures of the typical Fe-based NASICON-type cathode materials. (b) Abundance in Earth’s crust [111] and (c) prices [112] of elements. (d) Potential vs. specific capacity plots for Na4Fe3(PO4)2(P2O7) [113], Na2Fe2(SO4)3 [108], Na2FePO4F [105], and Na3Fe2(PO4)3 [101] normalized to the theoretical specific capacity per one-electron Fe2+ Fe3+ transition from experimental data.For the active electrochemical elements, 4d elements have also been reported, except for the above reported 3d transition metal element redox couples. In 2018, NaMo2(PO4)3 was confirmed to achieve stable electrochemical cycling based on the Mo3+/4+ redox couple [114] with a theoretical specific capacity of 98.2 mAh g-1 at an equilibrium potential of 2.45 V. In addition, the reversible reactions of redox couples such as Nb4+/5+ [115], Ti3+/4+ [116], Zr3+/4+ [117], and Cr3+/4+ [93] have also been reported in NASICON-type materials. However, the thermodynamic equilibrium potentials of the above compounds are either too low or too high to be used in cathode materials, whereas the relevant research is still in the initial stage.
4. Multi-electron transfer reaction
The low mass ratio of the transition metal means that a multi-electron transfer reaction is required to go beyond the electrochemical performance of LiFePO4 (LFP). We will reveal the issue of reported compounds and show the basic rule for designing high-capacity cathode materials around Na content, transition metal sites, and polyanion frameworks.
4.1 Na content and structural stability
The number of alkali metal sites in the NASICON structure is typically 1-4. Recently, researchers found that a new phase with a Na content of 5 will occur when the discharge potential is between 0 and 1 V [118], and the polyanion skeleton structure is unchanged. However, previous results have shown that the discharge voltage platform of the above structures is close to 0 V, which cannot be used as cathode materials. Therefore, we speculate that the highest Na content is 4 among NASICON-type cathode materials. Furthermore, Yan et al [78] confirmed that structural collapse occurs in Na0V2(PO4)2F3 when charged to 4.8 V. Similar phenomena have also been reported in other systems. Theoretical calculations also show that the skeleton structure of NASICON-type compounds is difficult to maintain due to the high formation energy when the Na content is lower than 1. The above finding shows that it is necessary to design a structure with a Na content close to 4 to ensure enough Na+ ions for extraction.
Notably, Liu et al [61] suggested that V5+ with a small ionic radius can diffuse to the Na1 site and induce kinetic hysteresis. According to the above result, the reversibility of the V4+/5+ redox couple can be realized when maintaining the occupancy of the Na1 site at high voltage. Recently, many works have focused on Na1 and Na2 sites for developing low-cost and high-energy-density V-based NASICON-type cathode materials [63, 119]. However, Na+ ion diffusion in NASICON frameworks is completed by the cooperation of the Na1 and Na2 sites, which means the Na+ in the Na1 and Na2 sites are dynamically evolving throughout the charging and discharging process. Therefore, some V-based NASICON compounds with a Na content of 4 (e.g. Na4VNi(PO4)3, etc) showed limited reversibility even if there was enough Na+ at the Na2 site in the pristine structure. Here, we pinpoint that rather than focusing on precisely controlling the Na+ content of Na1 and Na2 sites in the initial structure, it might be more necessary to ensure that Na1 is a thermodynamically/kinetically stable site in the entire voltage platform of the V4+/5+ redox couple. Meanwhile, blocking the migration channel of V5+ to the Na site is crucial too.
4.2 Selection of transition metal elements
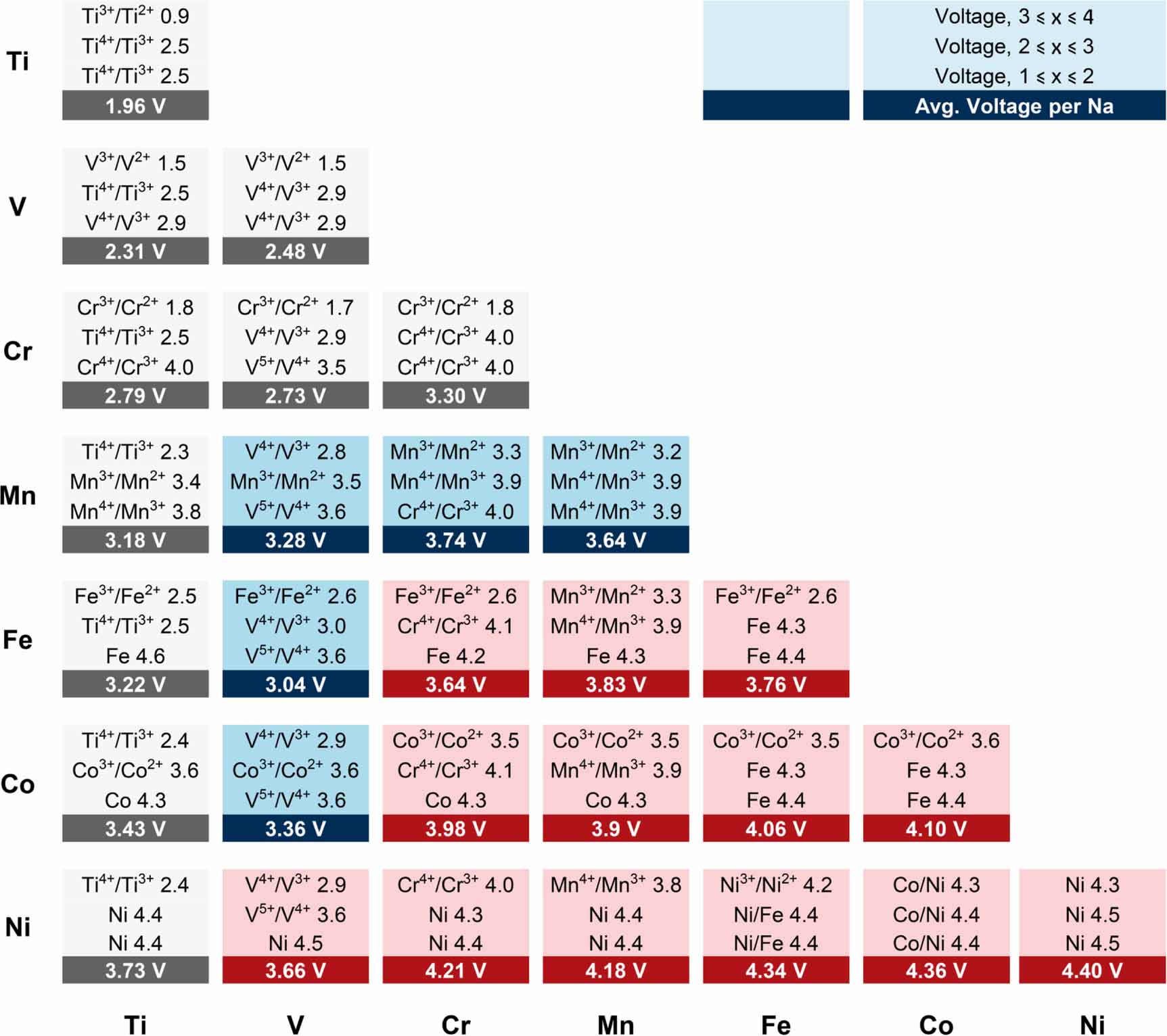
The elements that can be placed in transition metal sites are shown in figure 5. To facilitate element screening, we propose the following notes. First, the total valence state of the transition metal site is +5, and the Na content can be 4, so multi-element co-union is needed (M and M’ are +2 and +3, respectively). Second, previous studies have shown that V5+ easily migrates to the alkali sites, which may be the predominant issue for the irreversibility of V4+/5+ redox couples (e.g. Na4VMn(PO4)3, Na4VFe(PO4)3, Na4VNi(PO4)3, Na3VSc(PO4)3, etc). Interestingly, the substitution of Al3+ and Ga3+ in group 13 (IIIA) can realize the reversible reaction of the V4+/5+ redox couple [41, 63]. Therefore, it is essential to understand the relationship between the reversibility of the V4+/5+ redox couple and the NASICON backbone. Finally, Mn2+/4+ is a significant step in developing low-cost NASICON-type cathode materials. However, the activation of Mn2+/4+ redox couples often relies on Al3+, Ti4+, and Zr4+, which cannot be used for high energy density materials (the +4 valence state of Ti and Zr is too high, and Al3+ is electrochemically inactive) [24]. Excitingly, recent results have shown that Cr3+ can also activate the Mn2+/4+ redox couple, and the Cr3+/4+ redox reaction can be conducted at 4.4 V platform [93], suggesting a promising material that deep research is necessary to improve its electrochemical performance. Likewise, exploring other electrochemically active +3-valent elements for activating Mn2+/4+ is also a feasible strategy.
![]() Figure 5. A voltage map of NASICON electrodes, NaxMM(PO4)3, where M and M = Ti, V, Cr, Mn, Fe, Co, and Ni. The text in each box represents the redox couple and the corresponding voltage vs. Na+/Na [120]. The redox couples and the corresponding electrode potentials are shown in boxes (e.g. the redox couple in the range of 3 x 4 (x of NaxV2(PO4)3) is V2+/3+, and the electrode potential is 1.5 V). Reproduced from [120] with permission from the Royal Society of Chemistry.
Figure 5. A voltage map of NASICON electrodes, NaxMM(PO4)3, where M and M = Ti, V, Cr, Mn, Fe, Co, and Ni. The text in each box represents the redox couple and the corresponding voltage vs. Na+/Na [120]. The redox couples and the corresponding electrode potentials are shown in boxes (e.g. the redox couple in the range of 3 x 4 (x of NaxV2(PO4)3) is V2+/3+, and the electrode potential is 1.5 V). Reproduced from [120] with permission from the Royal Society of Chemistry.4.3 Polyanion frameworks
The polyanion groups are various, such as BO33-, CO32-, C2O42-, SiO44-, PO43-, SO42-, etc. Currently, the reported materials with excellent electrochemical performance are mainly phosphate-based compounds, and the exploration of other anionic groups is still limited. In addition, researchers demonstrated that F, Cl, etc, were able to replace the O sites, which endowed an abundant selection of polyanion frameworks. Therefore, except for focusing on the element replacement of NaxMM’(PO4)3, more polyanion frameworks also need to be explored.
5. Future perspectives
The low cost, wide distribution, and abundant reserves of sodium resources triggered the research of Na-ion batteries (NIBs) in the energy storage devices field. More notably, polyanionic-type NIB cathode materials are expected to meet the expansive demands for large-scale applications, benefitting from their long-term stability and high safety. Since our group first proposed the Mn-rich cathodes, several low cost NASICON cathodes with excellent cycling performance have been reported. However, the low transition metal mass fraction (for example, Fe is 35.4 wt% in LiFePO4, and V is 22.35 wt% in Na3V2(PO4)3) of the above compounds leads to a limited theoretical specific capacity. Additionally, the costs of the total batteries are much higher than the costs of the active materials as additional items, such as electrolytes, binders, casings, and even the electric battery management system, are included. Therefore, a higher capacity is needed for developing advanced polyanionic-type NIB cathode materials, which can further reduce the cost of inactive material. For the next generation of NASICON-type cathode materials, the low transition metal mass ratio means that the multi-electron transfer reaction is essential for high-capacity NASICON-type cathode materials.
Through extensive literature review, we demonstrate the key challenge of realizing the 1.5e-/TM transfer reaction and delivering design rules from Na content, transition metal sites, and polyanion frameworks. Fortunately, the flexible structure gives a promising future in designing multi-electron transfer reaction NASICON-type cathode materials. Although it is important to develop new materials, it is equally essential to focus on the failure mechanism of reported high-capacity systems (such as Na4VMn(PO4)3, Na4VFe(PO4)3, Na4MnCr(PO4)3, etc.). Overall, the characteristics of polyanionic compounds differ substantially from those of traditional layered oxide materials, and in-depth research is needed.
Acknowledgments: This work was supported by the National Key R&D Program of China (2022YFB3807800), National Natural Science Foundation (NSFC) of China (51725206, 52122214, 52002394, and 52072403), and Youth Innovation Promotion Association of the Chinese Academy of Sciences (2020006). -
Figure 1. (a) Timeline of the development of phosphate-based cathode materials. (b) Potential and specific capacity of different cathode materials. Squares are one-electron reactions of each transition metal (named 1e-/TM); the circle symbols are 1.5e-/TM. NOTE: the reactions with 1.5e-/TM in Na4VMn(PO4)3 and Na4VFe(PO4)3 are irreversible.
Figure 2. (a) Crystal structures and (b) voltage profiles of the typical V-based NASICON-type cathode materials, data from [69, 70, 79, 80]. (c) Charge curves of the V3+ to V5+ in which the V4+/5+ redox couple is irreversible, data from [54, 62]. (d) Voltage profiles of the reversible V4+/5+ redox couple reactions, data from [46].
Figure 4. (a) Crystal structures of the typical Fe-based NASICON-type cathode materials. (b) Abundance in Earth’s crust [111] and (c) prices [112] of elements. (d) Potential vs. specific capacity plots for Na4Fe3(PO4)2(P2O7) [113], Na2Fe2(SO4)3 [108], Na2FePO4F [105], and Na3Fe2(PO4)3 [101] normalized to the theoretical specific capacity per one-electron Fe2+ Fe3+ transition from experimental data.
Figure 5. A voltage map of NASICON electrodes, NaxMM(PO4)3, where M and M = Ti, V, Cr, Mn, Fe, Co, and Ni. The text in each box represents the redox couple and the corresponding voltage vs. Na+/Na [120]. The redox couples and the corresponding electrode potentials are shown in boxes (e.g. the redox couple in the range of 3 x 4 (x of NaxV2(PO4)3) is V2+/3+, and the electrode potential is 1.5 V). Reproduced from [120] with permission from the Royal Society of Chemistry.
-
[1] Grosjean C, Miranda P H, Perrin M, Poggi P 2012 Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry Renew. Sustain. Energy Rev. 16 1735-44 DOI: 10.1016/j.rser.2011.11.023
[2] Kesler S E, Gruber P W, Medina P A, Keoleian G A, Everson M P, Wallington T J 2012 Global lithium resources: relative importance of pegmatite, brine and other deposits Ore Geol. Rev. 48 55-69 DOI: 10.1016/j.oregeorev.2012.05.006
[3] Miedema J H, Moll H C 2013 Lithium availability in the EU27 for battery-driven vehicles: the impact of recycling and substitution on the confrontation between supply and demand until 2050 Resour. Policy 38 204-11 DOI: 10.1016/j.resourpol.2013.01.001
[4] Romero H, Mndez M, Smith P 2012 Mining development and environmental injustice in the Atacama desert of Northern Chile Environ. Justice 5 70-76 DOI: 10.1089/env.2011.0017
[5] Slater M D, Kim D, Lee E, Johnson C S 2013 Sodium-ion batteries Adv. Funct. Mater. 23 947-58 DOI: 10.1002/adfm.201200691
[6] Vaalma C, Buchholz D, Weil M, Passerini S 2018 A cost and resource analysis of sodium-ion batteries Nat. Rev. Mater. 3 1-11 DOI: 10.1038/natrevmats.2018.13
[7] Zhao C, et al 2020 Rational design of layered oxide materials for sodium-ion batteries Science 370 708-11 DOI: 10.1126/science.aay9972
[8] Zaghib K, Dub J, Dallaire A, Galoustov K, Guerfi A, Ramanathan M, Benmayza A, Prakash J, Mauger A, Julien C M 2012 Enhanced thermal safety and high power performance of carbon-coated LiFePO4 olivine cathode for Li-ion batteries J. Power Sources 219 36-44 DOI: 10.1016/j.jpowsour.2012.05.018
[9] Jin T, Li H, Zhu K, Wang P F, Liu P, Jiao L 2020 Polyanion-type cathode materials for sodium-ion batteries Chem. Soc. Rev. 49 2342-77 DOI: 10.1039/C9CS00846B
[10] Rajagopalan R, Zhang Z, Tang Y, Jia C, Ji X, Wang H 2021 Understanding crystal structures, ion diffusion mechanisms and sodium storage behaviors of NASICON materials Energy Storage Mater. 34 171-93 DOI: 10.1016/j.ensm.2020.09.007
[11] Anantharamulu N, Koteswara Rao K, Rambabu G, Vijaya Kumar B, Radha V, Vithal M 2011 A wide-ranging review on NASICON-type materials J. Mater. Sci. 46 2821-37 DOI: 10.1007/s10853-011-5302-5
[12] Chen S, Wu C, Shen L, Zhu C, Huang Y, Xi K, Maier J, Yu Y 2017 Challenges and perspectives for NASICON-type electrode materials for advanced sodium-ion batteries Adv. Mater. Weinheim 29 1700431 DOI: 10.1002/adma.201700431
[13] Hagman L-O, Kierkegaard P, Karvonen P, Virtanen A I, Paasivirta J 1968 The crystal structure of NaM2IV(PO43; MeIV = Ge, Ti, Zr Acta Chem. Scand. 22 1822-32 DOI: 10.3891/acta.chem.scand.22-1822
[14] Goodenough J B 1976 Hong HYP and Kafalas JA Fast Na+-ion transport in skeleton structures Mater. Res. Bull. 11 203-20 DOI: 10.1016/0025-5408(76)90077-5
[15] Hong H Y P 1976 Crystal structures and crystal chemistry in the system Na1+xZr2SixP3-xO12 Mater. Res. Bull. 11 173-82 DOI: 10.1016/0025-5408(76)90073-8
[16] Nadiri A, Delmas C, Salmon R, Hagenmuller P 1985 Chemical and electrochemical alkali metal intercalation in the iron(III) molybdate Fe2(MoO43 Chem. Inf.-Dienst 16 537-44 DOI: 10.1002/chin.198509038
[17] Delmas C, Nadiri A, Soubeyroux J L 1988 The NASICON-type titanium phosphates ATi2(PO43 (A=Li, Na) as electrode materials Solid State Ion. 28-30 419-23 DOI: 10.1016/S0167-2738(88)80075-4
[18] Uebou Y, Kiyabu T, Okada S, Yamaki J-I 2002 Electrochemical sodium insertion into the 3D-framework of Na3M2(PO43(M=Fe, V) Rep. Res. Inst. Appl. Mech. Kyushu Univ. 16 1-5
[19] Delmas C, Olazcuaga R, Cherkaoui F, Brochu R, Le Flem G 1978 A new family of phosphates with the formula Na3M2(PO43 (M = Ti, V, Cr, Fe) C. R. Acad. Sci. 10 168-74
[20] Jian Z, Zhao L, Pan H, Hu Y-S, Li H, Chen W, Chen L 2012 Carbon coated Na3V2(PO43 as novel electrode material for sodium ion batteries Electrochem. Commun. 14 86-89 DOI: 10.1016/j.elecom.2011.11.009
[21] Liang L, Li X, Zhao F, Zhang J, Liu Y, Hou L, Yuan C 2021 Construction and operating mechanism of high-rate Mo-doped Na3V2(PO43@C nanowires toward practicable wide-temperature-tolerance Na-ion and hybrid Li/Na-ion batteries Adv. Energy Mater. 11 2100287 DOI: 10.1002/aenm.202100287
[22] Xiong H, Sun G, Liu Z, Zhang L, Li L, Zhang W, Du F, Qiao Z A 2021 Polymer stabilized droplet templating towards tunable hierarchical porosity in single crystalline Na3V2(PO43 for enhanced sodium-ion storage Angew. Chem., Int. Ed. 60 10334-41 DOI: 10.1002/anie.202100954
[23] Vesborg P C K, Jaramillo T F 2012 Addressing the terawatt challenge: scalability in the supply of chemical elements for renewable energy RSC Adv. 2 7933-47 DOI: 10.1039/c2ra20839c
[24] Pan H, Hu Y-S, Chen L 2013 Room-temperature stationary sodium-ion batteries for large-scale electric energy storage Energy Environ. Sci. 6 2338-60 DOI: 10.1039/c3ee40847g
[25] Gao H, Li Y, Park K, Goodenough J B 2016 Sodium extraction from NASICON-structured Na3MnTi(PO43 through Mn(III)/Mn(II) and Mn(IV)/Mn(III) redox couples Chem. Mater. 28 6553-9 DOI: 10.1021/acs.chemmater.6b02096
[26] Kim H, Park I, Seo D-H, Lee S, Kim S-W, Kwon W J, Park Y-U, Kim C S, Jeon S, Kang K 2012 New iron-based mixed-polyanion cathodes for lithium and sodium rechargeable batteries: combined first principles calculations and experimental study J. Am. Chem. Soc. 134 10369-72 DOI: 10.1021/ja3038646
[27] Cao Y, Yang C, Liu Y, Xia X, Zhao D, Cao Y, Yang H, Zhang J, Lu J, Xia Y 2020 A new polyanion Na3Fe2(PO4)P2O7 cathode with high electrochemical performance for sodium-ion batteries ACS Energy Lett. 5 3788-96 DOI: 10.1021/acsenergylett.0c01902
[28] Park Y U, Seo D-H, Kim B, Hong K-P, Kim H, Lee S, Shakoor R A, Miyasaka K, Tarascon J-M, Kang K 2012 Tailoring a fluorophosphate as a novel 4 V cathode for lithium-ion batteries Sci. Rep. 2 704 DOI: 10.1038/srep00704
[29] Jian Z, et al 2014 Atomic structure and kinetics of NASICON NaxV2(PO43 cathode for sodium-ion batteries Adv. Funct. Mater. 24 4265-72 DOI: 10.1002/adfm.201400173
[30] Zou Z, et al 2021 Identifying migration channels and bottlenecks in monoclinic NASICONtype solid electrolytes with hierarchical iontransport algorithms Adv. Funct. Mater. 31 2107747 DOI: 10.1002/adfm.202107747
[31] Qiu Q, Li C, Liu H, Liao Y, Zhao C, Geng F, Shen M, Li J, Tong W, Hu B 2021 NMR evidence for the multielectron reaction mechanism of Na3V2(PO43 cathode and the impact of polyanion site substitution J. Phys. Chem. C 125 15200-9 DOI: 10.1021/acs.jpcc.1c04099
[32] Park S, Wang Z, Deng Z, Moog I, Canepa P, Fauth F, Carlier D, Croguennec L, Masquelier C, Chotard J-N 2021 Crystal structure of Na2V2(PO43, an intriguing phase spotted in the Na3V2(PO43-Na1V2(PO43 system Chem. Mater. 34 451-62 DOI: 10.1021/acs.chemmater.1c04033
[33] Li S, Dong Y, Xu L, Xu X, He L, Mai L 2014 Effect of carbon matrix dimensions on the electrochemical properties of Na3V2(PO43 nanograins for high-performance symmetric sodium-ion batteries Adv. Mater. Weinheim 26 3545-53 DOI: 10.1002/adma.201305522
[34] Zhu C, Song K, van Aken P A, Maier J, Yu Y 2014 Carbon-coated Na3V2(PO43 embedded in porous carbon matrix: an ultrafast Na-storage cathode with the potential of outperforming Li cathodes Nano Lett. 14 2175-80 DOI: 10.1021/nl500548a
[35] Dou X, Hasa I, Saurel D, Vaalma C, Wu L, Buchholz D, Bresser D, Komaba S, Passerini S 2019 Hard carbons for sodium-ion batteries: structure, analysis, sustainability, and electrochemistry Mater. Today 23 87-104 DOI: 10.1016/j.mattod.2018.12.040
[36] Oh J A S, He H, Sun J, Cao X, Chua B, Huang Y, Zeng K, Lu L 2020 Dual-nitrogen-doped carbon decorated on Na3V2(PO43 to stabilize the intercalation of three sodium ions ACS Appl. Energy Mater. 3 6870-9 DOI: 10.1021/acsaem.0c00973
[37] Yi G-D, Fan C-L, Hu Z, Zhang W-H, Han S-C, Liu J-S 2021 Construction of high performance N-doped Na3V2(PO42F3/C cathode assisting by plasma enhanced chemical vapor deposition for sodium-ion batteries Electrochim. Acta 383 138370 DOI: 10.1016/j.electacta.2021.138370
[38] An Q, Xiong F, Wei Q, Sheng J, He L, Ma D, Yao Y, Mai L 2015 Nanoflake-assembled hierarchical Na3V2(PO43/C microflowers: superior Li storage performance and insertion/extraction mechanism Adv. Energy Mater. 5 1401963 DOI: 10.1002/aenm.201401963
[39] Yan J, Yuan W, Tang Z-Y, Xie H, Mao W-F, Ma L 2012 Synthesis and electrochemical performance of Li3V2(PO43-xClx/C cathode materials for lithium-ion batteries J. Power Sources 209 251-6 DOI: 10.1016/j.jpowsour.2012.02.110
[40] Peng M, Zhang D, Zheng L, Wang X, Lin Y, Xia D, Sun Y, Guo G 2017 Hierarchical Ru-doped sodium vanadium fluorophosphates hollow microspheres as a cathode of enhanced superior rate capability and ultralong stability for sodium-ion batteries Nano Energy 31 64-73 DOI: 10.1016/j.nanoen.2016.11.023
[41] Hu Q, Liao J-Y, He X-D, Wang S, Xiao L-N, Ding X, Chen C-H 2019 In situ catalytic formation of graphene-like graphitic layer decoration on Na3V2-xGax(PO43 (0 x 0.6) for ultrafast and high energy sodium storage J. Mater. Chem. A 7 4660-7 DOI: 10.1039/C8TA11890F
[42] Bi L, Liu X, Li X, Chen B, Zheng Q, Xie F, Huo Y, Lin D 2020 Modulation of the crystal structure and ultralong life span of a Na3V2(PO43-based cathode for a high-performance sodium-ion battery by niobium-vanadium substitution Ind. Eng. Chem. Res. 59 21039-46 DOI: 10.1021/acs.iecr.0c04187
[43] Park J Y, et al 2020 An iron-doped NASICON type sodium ion battery cathode for enhanced sodium storage performance and its full cell applications J. Mater. Chem. A 8 20436-45 DOI: 10.1039/D0TA07766F
[44] Chen Y, Cheng J, Sun S, Tian Z, Jiang X, Wang Y, He Z, Liu C, Huang Q, Guo L 2021 Constructing hierarchical porous Fe/F-codoped Na3V2(PO43/C composite enwrapped with carbon nanotubes as high-performance cathode for symmetric sodium ion batteries J. Power Sources 513 230545 DOI: 10.1016/j.jpowsour.2021.230545
[45] Ghosh S, Jose N, Senthilkumar B, Amonpattaratkit P, Senguttuvan P 2021 Multi-redox (V5+/V4+/V3+/V2+) driven asymmetric sodium (de)intercalation reactions in NASICON-Na3VIn(PO43 cathode J. Electrochem. Soc. 168 050534 DOI: 10.1149/1945-7111/ac001d
[46] Liu R, et al 2017 Exploring highly reversible 1.5-electron reactions (V3+/V4+/V5+) in Na3VCr(PO43 cathode for sodium-ion batteries ACS Appl. Mater. Interfaces 9 43632-9 DOI: 10.1021/acsami.7b13018
[47] Gao H, Seymour I D, Xin S, Xue L, Henkelman G, Goodenough J B 2018 Na3MnZr(PO43: a high-voltage cathode for sodium batteries J. Am. Chem. Soc. 140 18192-9 DOI: 10.1021/jacs.8b11388
[48] Zhou W, Xue L, L X, Gao H, Li Y, Xin S, Fu G, Cui Z, Zhu Y, Goodenough J B 2016 NaxMV(PO43 (M = Mn, Fe, Ni) structure and properties for sodium extraction Nano Lett. 16 7836-41 DOI: 10.1021/acs.nanolett.6b04044
[49] Zhang X, Rui X, Chen D, Tan H, Yang D, Huang S, Yu Y 2019 Na3V2(PO43: an advanced cathode for sodium-ion batteries Nanoscale 11 2556-76 DOI: 10.1039/C8NR09391A
[50] Boivin E, Chotard J N, Masquelier C, Croguennec L 2021 Towards reversible high-voltage multi-electron reactions in alkali-ion batteries using vanadium phosphate positive electrode materials Molecules 26 1428 DOI: 10.3390/molecules26051428
[51] Lv Z, Ling M, Yue M, Li X, Song M, Zheng Q, Zhang H 2021 Vanadium-based polyanionic compounds as cathode materials for sodium-ion batteries: toward high-energy and high-power applications J. Energy Chem. 55 361-90 DOI: 10.1016/j.jechem.2020.07.008
[52] Chotard J-N, Rousse G, David R, Mentr O, Courty M, Masquelier C 2015 Discovery of a sodium-ordered form of Na3V2(PO43 below ambient temperature Chem. Mater. 27 5982-7 DOI: 10.1021/acs.chemmater.5b02092
[53] Rui X, Sun W, Wu C, Yu Y, Yan Q 2015 An advanced sodium-ion battery composed of carbon coated Na3V2(PO43 in a porous graphene network Adv. Mater. Weinheim 27 6670-6 DOI: 10.1002/adma.201502864
[54] Chen F, Kovrugin V M, David R, Mentr O, Fauth F, Chotard J N, Masquelier C 2018 A NASICONtype positive electrode for Na batteries with high energy density: Na4MnV(PO43 Small Methods 3 1800218 DOI: 10.1002/smtd.201800218
[55] Ghosh S, Barman N, Mazumder M, Pati S K, Rousse G, Senguttuvan P 2019 High capacity and high-rate NASICON-Na3.75V1.25Mn0.75(PO43 cathode for Na-ion batteries via modulating electronic and crystal structures Adv. Energy Mater. 10 1902918 DOI: 10.1002/aenm.201902918
[56] Anishchenko D V, Zakharkin M V, Nikitina V A, Stevenson K J, Antipov E V 2020 Phase boundary propagation kinetics predominately limit the rate capability of NASICON-type Na3+xMnxV2-x(PO43 (0x1) materials Electrochim. Acta 354 136761 DOI: 10.1016/j.electacta.2020.136761
[57] Ghosh S, Barman N, Senguttuvan P 2020 Impact of Mg2+ and Al3+ substitutions on the structural and electrochemical properties of NASICONNaxVMn0. 75M0. 25(PO43 (M= Mg and Al) cathodes for sodiumion batteries Small 16 e2003973 DOI: 10.1002/smll.202003973
[58] Ma X, Cao X, Zhou Y, Guo S, Shi X, Fang G, Pan A, Lu B, Zhou J, Liang S 2020 Tuning crystal structure and redox potential of NASICON-type cathodes for sodium-ion batteries Nano Res. 13 3330-7 DOI: 10.1007/s12274-020-3011-6
[59] Xu C, et al 2021 A novel NASICONtyped Na4VMn0.5Fe0.5(PO43 cathode for highperformance Naion batteries Adv. Energy Mater. 11 2100729 DOI: 10.1002/aenm.202100729
[60] Zhang J, Zhao X, Song Y, Li Q, Liu Y, Chen J, Xing X 2019 Understanding the superior sodium-ion storage in a novel Na3.5Mn0.5V1.5(PO43 cathode Energy Stor. Mater. 23 25-34 DOI: 10.1016/j.ensm.2019.05.041
[61] Liu R, Zheng S, Yuan Y, Yu P, Liang Z, Zhao W, ShahbazianYassar R, Ding J, Lu J, Yang Y 2020 Counter-intuitive structural instability aroused by transition metal migration in polyanionic sodium ion host Adv. Energy Mater. 11 2003256 DOI: 10.1002/aenm.202003256
[62] Perfilyeva T I, et al 2021 Complete three-electron vanadium redox in NASICON-type Na3VSc(PO43 electrode material for Na-ion batteries J. Electrochem. Soc. 168 110550 DOI: 10.1149/1945-7111/ac393d
[63] Wang Q, Gao H, Li J, Liu G B, Jin H 2021 Importance of crystallographic sites on sodium-ion extraction from NASICON-structured cathodes for sodium-ion batteries ACS Appl. Mater. Interfaces 13 14312-20 DOI: 10.1021/acsami.1c01663
[64] Sun C, Zhao Y, Ni Q, Sun Z, Yuan X, Li J, Jin H 2022 Reversible multielectron redox in NASICON cathode with high energy density for low-temperature sodium-ion batteries Energy Stor. Mater. 49 291-8 DOI: 10.1016/j.ensm.2022.04.025
[65] Li M, Sun C, Ni Q, Sun Z, Liu Y, Li Y, Li L, Jin H, Zhao Y 2023 High entropy enabling the reversible redox reaction of V4+/V5+ couple in NASICONtype sodium ion cathode Adv. Energy Mater. 13 2203971 DOI: 10.1002/aenm.202203971
[66] Zhao Y, Gao X, Gao H, Jin H, Goodenough J B 2020 Three electron reversible redox reaction in sodium vanadium chromium phosphate as a high-energy-density cathode for sodium-ion batteries Adv. Funct. Mater. 30 1908680 DOI: 10.1002/adfm.201908680
[67] Liu Y, Sun C, Ni Q, Sun Z, Li M, Ma S, Jin H, Zhao Y 2022 Enhanced electrochemical performance of NASICON-type sodium ion cathode based on charge balance theory Energy Stor Mater. 53 881-9 DOI: 10.1016/j.ensm.2022.10.011
[68] Liu Y, Li J, Shen Q, Zhang J, He P, Qu X, Liu Y 2022 Advanced characterizations and measurements for sodium-ion batteries with NASICON-type cathode materials eScience 2 10-31 DOI: 10.1016/j.esci.2021.12.008
[69] Ling M, Lv Z, Li F, Zhao J, Zhang H, Hou G, Zheng Q, Li X 2020 Revisiting of tetragonal NaVPO4F: a high energy density cathode for sodium-ion batteries ACS Appl. Mater. Interfaces 12 30510-9 DOI: 10.1021/acsami.0c08846
[70] Shraer S D, et al 2022 Development of vanadium-based polyanion positive electrode active materials for high-voltage sodium-based batteries Nat. Commun. 13 4097 DOI: 10.1038/s41467-022-31768-5
[71] Le Meins J M, Crosnier-Lopez M P, Hemon-Ribaud A, Courbion G 1999 Phase transitions in the Na3M2(PO42F3 family (M=Al3+, V3+, Cr3+, Fe3+, Ga3+): synthesis, thermal, structural, and magnetic studies J. Solid State Chem. 148 260-77 DOI: 10.1006/jssc.1999.8447
[72] Gover R, Bryan A, Burns P, Barker J 2006 The electrochemical insertion properties of sodium vanadium fluorophosphate, Na3V2(PO42F3 Solid State Ion. 177 1495-500 DOI: 10.1016/j.ssi.2006.07.028
[73] Xu M, Xiao P, Stauffer S, Song J, Henkelman G, Goodenough J B 2014 Theoretical and experimental study of vanadium-based fluorophosphate cathodes for rechargeable batteries Chem. Mater. 26 3089-97 DOI: 10.1021/cm500106w
[74] Dacek S T, Richards W D, Kitchaev D A, Ceder G 2016 Structure and dynamics of fluorophosphate Na-ion battery cathodes Chem. Mater. 28 5450-60 DOI: 10.1021/acs.chemmater.6b01989
[75] Massa W, Yakubovich O V, Dimitrova O V 2002 Crystal structure of a new sodium vanadyl(IV) fluoride phosphate Na3{V2O2F[PO42} Solid State Sci. 4 495-501 DOI: 10.1016/S1293-2558(02)01283-9
[76] Park Y U, Seo D H, Kwon H S, Kim B, Kim J, Kim H, Kim I, Yoo H I, Kang K 2013 A new high-energy cathode for a Na-ion battery with ultrahigh stability J. Am. Chem. Soc. 135 13870-8 DOI: 10.1021/ja406016j
[77] Kumar P R, Jung Y H, Lim C H, Kim D K 2015 Na3V2O2x(PO42F3-2x: a stable and high-voltage cathode material for aqueous sodium-ion batteries with high energy density J. Mater. Chem. A 3 6271-5 DOI: 10.1039/C5TA00980D
[78] Yan G, Mariyappan S, Rousse G, Jacquet Q, Deschamps M, David R, Mirvaux B, Freeland J W, Tarascon J M 2019 Higher energy and safer sodium ion batteries via an electrochemically made disordered Na3V2(PO42F3 material Nat. Commun. 10 585 DOI: 10.1038/s41467-019-08359-y
[79] Bianchini M, Fauth F, Brisset N, Weill F, Suard E, Masquelier C, Croguennec L 2015 Comprehensive investigation of the Na3V2(PO42F3-NaV2(PO42F3 system by operando high resolution synchrotron x-ray diffraction Chem. Mater. 27 3009-20 DOI: 10.1021/acs.chemmater.5b00361
[80] Guo J Z, Wang P F, Wu X L, Zhang X H, Yan Q, Chen H, Zhang J P, Guo Y G 2017 High-energy/power and low-temperature cathode for sodium-ion batteries: in situ XRD study and superior full-cell performance Adv. Mater. Weinheim 29 1701968 DOI: 10.1002/adma.201701968
[81] Snarskis G, Pilipavicius J, Gryaznov D, Mikoliu Naite L, Vilciauskas L 2021 Peculiarities of phase formation in Mn-based Na superIonic conductor (NaSICon) systems: the case of Na1+2xMnxTi2-x(PO43 (0.0 x 1 5 Chem. Mater. 33 8394-403 DOI: 10.1021/acs.chemmater.1c02775
[82] Zhou Y, Shao X, Lam K H, Zheng Y, Zhao L, Wang K, Zhao J, Chen F, Hou X 2020 Symmetric sodium-ion battery based on dual-electron reactions of NASICON-structured Na3MnTi(PO43 material ACS Appl. Mater. Interfaces 12 30328-35 DOI: 10.1021/acsami.0c05784
[83] Liu J, Lin K, Zhao Y, Zhou Y, Hou X, Liu X, Lou H, Lam K-H, Chen F 2021 Exceeding three-electron reactions in Na3+2xMn1+xTi1-x(PO43 NASICON cathodes with high energy density for sodium-ion batteries J. Mater. Chem. A 9 10437-46 DOI: 10.1039/D1TA01148K
[84] Li H, Xu M, Gao C, Zhang W, Zhang Z, Lai Y, Jiao L 2020 Highly efficient, fast and reversible multi-electron reaction of Na3MnTi(PO43 cathode for sodium-ion batteries Energy Storage Mater. 26 325-33 DOI: 10.1016/j.ensm.2019.11.004
[85] Zhu T, et al 2019 Realizing three-electron redox reactions in NASICON-structured Na3MnTi(PO43 for sodium-ion batteries Adv. Energy Mater. 9 2338-60 DOI: 10.1002/aenm.201803436
[86] Zhu T, Hu P, Cai C, Liu Z, Hu G, Kuang Q, Mai L, Zhou L 2020 Dual carbon decorated Na3MnTi(PO43: a high-energy-density cathode material for sodium-ion batteries Nano Energy 70 104548 DOI: 10.1016/j.nanoen.2020.104548
[87] Sun X, Wang T, Zhang W, Li H, Lai Y, Zhang Z 2020 Dual carbon decorated Na3MnTi(PO43 as an advanced cathode for sodium-ion batteries Ionics 26 3919-27 DOI: 10.1007/s11581-020-03538-0
[88] Gao H, Goodenough J B 2016 An aqueous symmetric sodium-ion battery with NASICON-structured Na3MnTi(PO43 Angew. Chem., Int. Ed. 55 12768-72 DOI: 10.1002/anie.201606508
[89] Zhang J, Lin C, Xia Q, Wang C, Zhao X S 2021 Improved performance of Na3MnTi(PO43 using a non-stoichiometric synthesis strategy ACS Energy Lett. 6 2081-9 DOI: 10.1021/acsenergylett.1c00426
[90] Ma X, Wu X, Liu Y, Wu W, Pan Z, Shen P K 2021 Toward a high-energy-density cathode with enhanced temperature adaptability for sodium-ion batteries: a case study of Na3MnZr(PO43 microspheres with embedded dual-carbon networks ACS Appl. Mater. Interfaces 13 21390-400 DOI: 10.1021/acsami.1c03642
[91] Zhang J, Liu Y, Zhao X, He L, Liu H, Song Y, Sun S, Li Q, Xing X, Chen J 2020 A novel NASICON-type Na4MnCr(PO43 demonstrating the energy density record of phosphate cathodes for sodium-ion batteries Adv. Mater. Weinheim 32 1906348 DOI: 10.1002/adma.201906348
[92] Zhang W, Li H, Zhang Z, Xu M, Lai Y, Chou S L 2020 Full activation of Mn4+/Mn3+ redox in Na4MnCr(PO43 as a high-voltage and high-rate cathode material for sodium-ion batteries Small 16 e2001524 DOI: 10.1002/smll.202001524
[93] Wang J, Wang Y, Seo D H, Shi T, Chen S, Tian Y, Kim H, Ceder G 2020 A high-energy NASICON-type cathode material for Na-ion batteries Adv. Energy Mater. 10 1903968 DOI: 10.1002/aenm.201903968
[94] Zhao Y, Gao X, Gao H, Dolocan A, Goodenough J B 2021 Elevating energy density for sodium-ion batteries through multielectron reactions Nano Lett. 21 2281-7 DOI: 10.1021/acs.nanolett.1c00100
[95] Li J, et al 2022 Stabilized multi-electron reactions in a high-energy Na4Mn0.9CrMg0.1(PO43 sodium-storage cathode enabled by the pinning effect Small 18 e2202879 DOI: 10.1002/smll.202202879
[96] Wang Q, Ling C, Li J, Gao H, Wang Z, Jin H 2021 Experimental and theoretical investigation of Na4MnAl(PO43 cathode material for sodium-ion batteries Chem. Eng. J. 425 130680 DOI: 10.1016/j.cej.2021.130680
[97] He L, Li H, Ge X, Li S, Wang X, Wang S, Zhang L, Zhang Z 2022 Ironphosphatebased cathode materials for costeffective sodiumion batteries: development, challenges, and prospects Adv. Mater. Interfaces 9 2200515 DOI: 10.1002/admi.202200515
[98] Avdeev M, Mohamed Z, Ling C D, Lu J, Tamaru M, Yamada A, Barpanda P 2013 Magnetic structures of NaFePO4 maricite and triphylite polymorphs for sodium-ion batteries Inorg. Chem. 52 8685-93 DOI: 10.1021/ic400870x
[99] Cao Y J, Liu Y, Zhao D Q, Xia X P, Zhang L C, Zhang J X, Yang H S, Xia Y Y 2020 Highly stable Na3Fe2(PO43@hard carbon sodium-ion full cell for low-cost energy storage ACS Sustain. Chem. Eng. 8 1380-7 DOI: 10.1021/acssuschemeng.9b05098
[100] Qiu S, et al 2019 NASICON-type Na3Fe2(PO43 as a low-cost and high-rate anode material for aqueous sodium-ion batteries Na3Fe2(PO43 as a low-cost and high-rate anode material for aqueous sodium-ion batteries Nano Energy 64 103941 DOI: 10.1016/j.nanoen.2019.103941
[101] Rajagopalan R, et al 2017 Improved reversibility of Fe3+/Fe4+ redox couple in sodium super ion conductor type Na3Fe2(PO43 for sodium-ion batteries Adv. Mater. Weinheim 29 1605694 DOI: 10.1002/adma.201605694
[102] Barpanda P, Liu G D, Ling C D, Tamaru M, Avdeev M, Chung S C, Yamada Y, Yamada A 2013 Na2FeP2O7: a safe cathode for rechargeable sodium-ion batteries Chem. Mater. 25 3480-7 DOI: 10.1021/cm401657c
[103] Jung Y H, Lim C H, Kim J H, Kim D K 2014 Na2FeP2O7 as a positive electrode material for rechargeable aqueous sodium-ion batteries RSC Adv. 4 9799-802 DOI: 10.1039/c3ra47560c
[104] Barpanda P, Ye T, Nishimura S-I, Chung S-C, Yamada Y, Okubo M, Zhou H, Yamada A 2012 Sodium iron pyrophosphate: a novel 3.0 V iron-based cathode for sodium-ion batteries Electrochem. Commun. 24 116-9 DOI: 10.1016/j.elecom.2012.08.028
[105] Li H, Wang T, Wang S, Wang X, Xie Y, Hu J, Lai Y, Zhang Z 2021 Scalable synthesis of the Na2FePO4F cathode through an economical and reliable approach for sodium-ion batteries ACS Sustain. Chem. Eng. 9 11798-806 DOI: 10.1021/acssuschemeng.1c03355
[106] Dong J, et al 2022 Electronic structure regulation of Na2FePO4F cathode toward superior high-rate and high-temperature sodium-ion batteries Energy Stor. Mater. 45 851-60 DOI: 10.1016/j.ensm.2021.12.034
[107] Barpanda P, Oyama G, Nishimura S, Chung S C, Yamada A 2014 A 3.8-V earth-abundant sodium battery electrode Nat. Commun. 5 4358 DOI: 10.1038/ncomms5358
[108] Chen M, et al 2018 A novel graphene oxide wrapped Na2Fe2(SO43/C cathode composite for long life and high energy density sodiumion batteries Adv. Energy Mater. 8 1800944 DOI: 10.1002/aenm.201800944
[109] Plewa A, Kulka A, Hanc E, Zajc W, Sun J, Lu L, Molenda J 2020 Facile aqueous synthesis of high performance Na2FeM(SO43 (M = Fe, Mn, Ni) alluaudites for low cost Na-ion batteries J. Mater. Chem. A 8 2728-40 DOI: 10.1039/C9TA11565J
[110] Wang H, Pan Z, Zhang H, Dong C, Ding Y, Cao Y, Chen Z 2021 A green and scalable synthesis of Na3Fe2(PO4)P2O7/rGO cathode for high-rate and long-life sodium-ion batteries Small Methods 5 e2100372 DOI: 10.1002/smtd.202100372
[111] William M H, David R L, Thomas J B 2016 CRC Handbook of Chemistry and Physics; Abundance of Elements in the Earth’s Crust and in the Sea97th edn (Boca Raton, FL: CRC Press) 14-17
[112] Trading economics 2022 (available at: https://tradingeconomics.com/)
[113] Chen M, et al 2019 NASICON-type air-stable and all-climate cathode for sodium-ion batteries with low cost and high-power density Nat. Commun. 10 1480 DOI: 10.1038/s41467-019-09170-5
[114] Panin R V, Drozhzhin O A, Fedotov S S, Khasanova N R, Antipov E V 2018 NASICON-type NaMo2(PO43: electrochemical activity of the Mo+4 polyanion compound in Na-cell Electrochim. Acta 289 168-74 DOI: 10.1016/j.electacta.2018.09.045
[115] Tillement O, Couturier J C, Angenault J, Quarton M 1991 Crystal chemistry and electrical study of NaxNbTi(PO43 Solid State Ion. 48 249-55 DOI: 10.1016/0167-2738(91)90039-E
[116] Tillement O, Angenault J, Couturier J C, Quarton M 1992 Electrochemical studies of mixed valence NASICON Solid State Ion. 53-56 391-9 DOI: 10.1016/0167-2738(92)90405-E
[117] Wang W, Jiang B, Hu L, Jiao S 2014 NASICON material NaZr2(PO43: a novel storage material for sodium-ion batteries J. Mater. Chem. A 2 1341-5 DOI: 10.1039/C3TA14310D
[118] Jian Z, Sun Y, Ji X 2015 A new low-voltage plateau of Na3V2(PO43 as an anode for Na-ion batteries Chem. Commun. 51 6381-3 DOI: 10.1039/C5CC00944H
[119] Xu C, et al 2022 Reversible activation of V4+/V5+ redox couples in NASICON phosphate cathodes Adv. Energy Mater. 12 2200966 DOI: 10.1002/aenm.202200966
[120] Singh B, Wang Z, Park S, Gautam G S, Chotard J-N, Croguennec L, Carlier D, Cheetham A K, Masquelier C, Canepa P 2021 A chemical map of NASICON electrode materials for sodium-ion batteries J. Mater. Chem. A 9 281-92 DOI: 10.1039/D0TA10688G
-
期刊类型引用(9)
1. Lu, F., Lu, Y., Zhao, L. Dual carbon decorated Na3.5Mn0.5V1.5(PO4)3 cathode with high-density and long-cycling span-life for sodium-ion batteries. Journal of Power Sources, 2025.  必应学术
必应学术
2. Salgado, R., Terny, S., Frechero, M.A. Structural, morphological, and electric study of doped- Na2Zn2TeO6 family in a wide range of temperatures. Materials Science and Engineering: B, 2025.  必应学术
必应学术
3. Guo, D., Chu, S., Zhang, B. et al. The Development and Prospect of Stable Polyanion Compound Cathodes in LIBs and Promising Complementers. Small Methods, 2024, 8(12): 2400587.  必应学术
必应学术
4. Wang, M., Zhu, H., Xue, Y. et al. Baiting bacteria with amino acidic and peptidic corona coated defect-engineered antimicrobial nanoclusters for optimized wound healing. Bioactive Materials, 2024.  必应学术
必应学术
5. Mukkattu Kuniyil, N.C., Robin, R., Gokulnath, S. et al. Nanoarchitectonic Approach to Zinc-Substituted NaSICON Na4VMn1-xZnx(PO4)3/C as High-Rate Cathodes for Sodium-Ion Batteries. Energy and Fuels, 2024, 38(17): 17026-17037.  必应学术
必应学术
6. Panin, R.V., Cherkashchenko, I.R., Zaitseva, V.V. et al. Realizing Three-Electron Redox Reactions in NASICON-Type NaCrNb(PO4)3 for Sodium Ion Battery Applications. Chemistry of Materials, 2024, 36(14): 6902-6911.  必应学术
必应学术
7. Zhang, X., Xu, C., Bai, Y. et al. NaVPO4X (X = O, F) as cathodes for advanced high-energy Na-ion batteries. Chemical Engineering Journal, 2024.  必应学术
必应学术
8. Conti, D.M., Urru, C., Bruni, G. et al. Design of Na3MnZr(PO4)3/Carbon Nanofiber Free-Standing Cathodes for Sodium-Ion Batteries with Enhanced Electrochemical Performances through Different Electrospinning Approaches. Molecules, 2024, 29(8): 1885.  必应学术
必应学术
9. Liu, Z., Cao, Y., Zhang, H. et al. Towards High-Performance Sodium-Ion Batteries via the Phase Regulation Strategy. ACS Sustainable Chemistry and Engineering, 2024, 12(2): 1132-1141.  必应学术
必应学术
其他类型引用(0)
-
其他相关附件
-
本文图文摘要
点击下载
-



 下载:
下载: