| Citation: | Hongmei Tang, Zhe Qu, Yaping Yan, Wenlan Zhang, Hua Zhang, Minshen Zhu, Oliver G Schmidt. Unleashing energy storage ability of aqueous battery electrolytes[J]. Materials Futures, 2022, 1(2): 022001. DOI: 10.1088/2752-5724/ac52e8 |

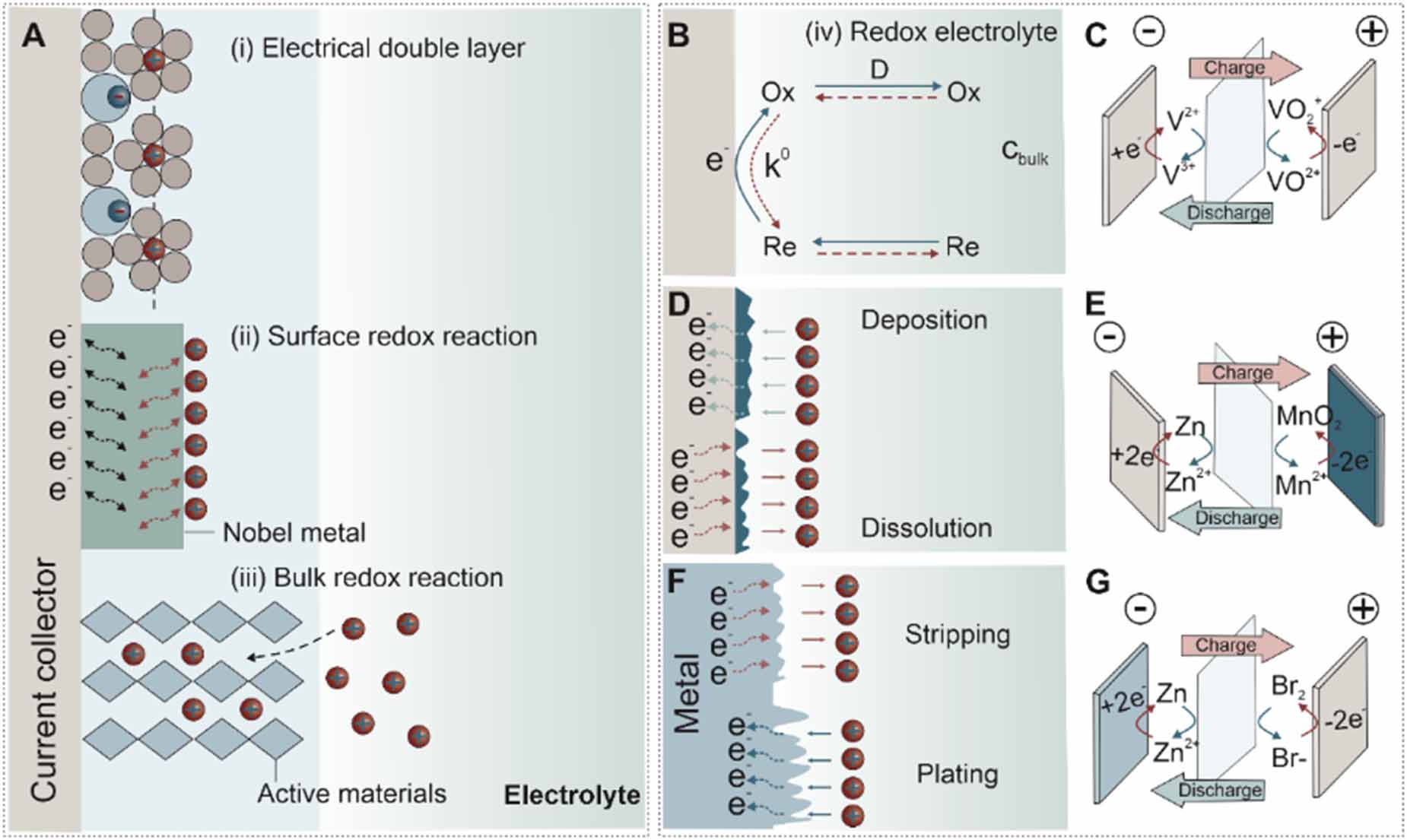
Electrochemical energy storage (EES) devices sit at the heart of the de-fossilization strategy towards carbon neutrality because it is essential to store energy from intermittent renewable sources (e.g. solar and wind) [1-3]. Among various EES devices, supercapacitors are able to operate at a high specific power (>10 kW kg-1), delivering the stored energy within tens of seconds and lasting for over 105 charge/discharge cycles [4]. Their charge storage mechanisms, electrical double-layer (EDL) formation and surface redox reactions, enable these particular properties of supercapacitors [5]. The EDL is formed (type-i in figure 1(A)) upon the physical adsorption of charged ions in the electrolyte on the electrode surface. The reversible sorption of dissolved ions in the electrolyte is a fast and non-Faradaic process, avoiding any significant structural variation and allowing for high-power capability and cycling stability. Porous carbonaceous materials are widely used as electrodes in electrical double-layer capacitors (EDLCs) owing to their large surface area providing plenty of adsorption sites [6]. In addition to EDLCs, pseudocapacitive electrode materials store charge via Faradaic processes based on fast and reversible redox reactions at the surface or near-surface positions (type-ii in figure 1(A)). The main disadvantage of supercapacitors is their limited energy density (<10 Wh kg-1). By contrast, batteries show a much-improved energy density of 300 Wh kg-1 [4, 7]. The trade-offs of the high energy density are short cycle life, a few thousand cycles for instance, and lower power density. Such differences against supercapacitors originate from the slow charge storage mechanism based on bulk redox reactions relying on the solid-state diffusion of ions. The structural changes of the bulk electrode materials build up and eventually cause battery failure. The intercalation pseudocapacitor is a promising design to combine the advantage of supercapacitors and batteries. Like lithium-ion batteries, ions dissolved in the electrolyte (e.g. Li+, Na+, K+, and H+) and also intercalate into the bulk electrode materials (type-iii in figure 1(A)) but with fast kinetics [8, 9].
The electrolyte in the above EES devices only acts as a medium to transfer and balance charges between electrodes and therefore does not contribute to energy storage. As such, the thinner the electrolyte layer is, the higher energy density can be achieved for EES devices. Alternatively, redox-active species dissolved in the electrolyte can substitute the function of electrode materials to store charges by redox reactions at the solid-liquid interface (figure 1(B)). Redox flow designs are an excellent approach to demonstrate the charge storage in the electrolyte. For instance, vanadium (V) and oxidized vanadium ions are used in the redox flow battery. Upon charging, VO2+ ions are oxidized to VO2+, and V3+ is reduced to form V2+. Inversely, VO2+ is reduced to VO2+, and V2+ is oxidized to V3+ during discharge (figure 1(C)) [10]. The redox flow batteries usually need electrolyte tanks to refill and circulate electrolytes. The same strategy is adopted at small scale in cathode-less (figure 1(D)) and anode-less (figure 1(F)) designs. For instance, MnO2 solids are deposited on the current collector by oxidizing soluble Mn2+ ions in the electrolyte (figure 1(E)). The reversible reaction between Mn2+ and MnO2 allows for the design of a secondary battery. The same principle applies to the in-situ formation of anode materials. Zn can be deposited on a conductive substrate under a negative potential (figure 1(G)).
By unleashing the energy storage ability of electrolytes, it is possible to substantially improve the energy density of batteries and open a new world for developing energy-dense and small-scale batteries. This article reviews the electrochemical basics of redox-active electrolytes and introduces representative redox-active species for diverse battery designs. Last but not least, we highlight the pioneering works on the concept of storing energy in the electrolyte and discuss the perspectives of batteries based on redox-active electrolytes.
Electrolytes need to exhibit high ionic conductivity, wide electrochemical windows, and high chemical and thermal stability. For redox-active electrolytes, a large potential difference between redox couples and multiple electron transfer of redox reactions are required to reach a high energy density [11]. At the device level, a high concentration of redox couples can improve the energy storage ability of the full cell. In practice, the electrochemical window, redox couples, and kinetics in mass and electron transport are three basic parameters.
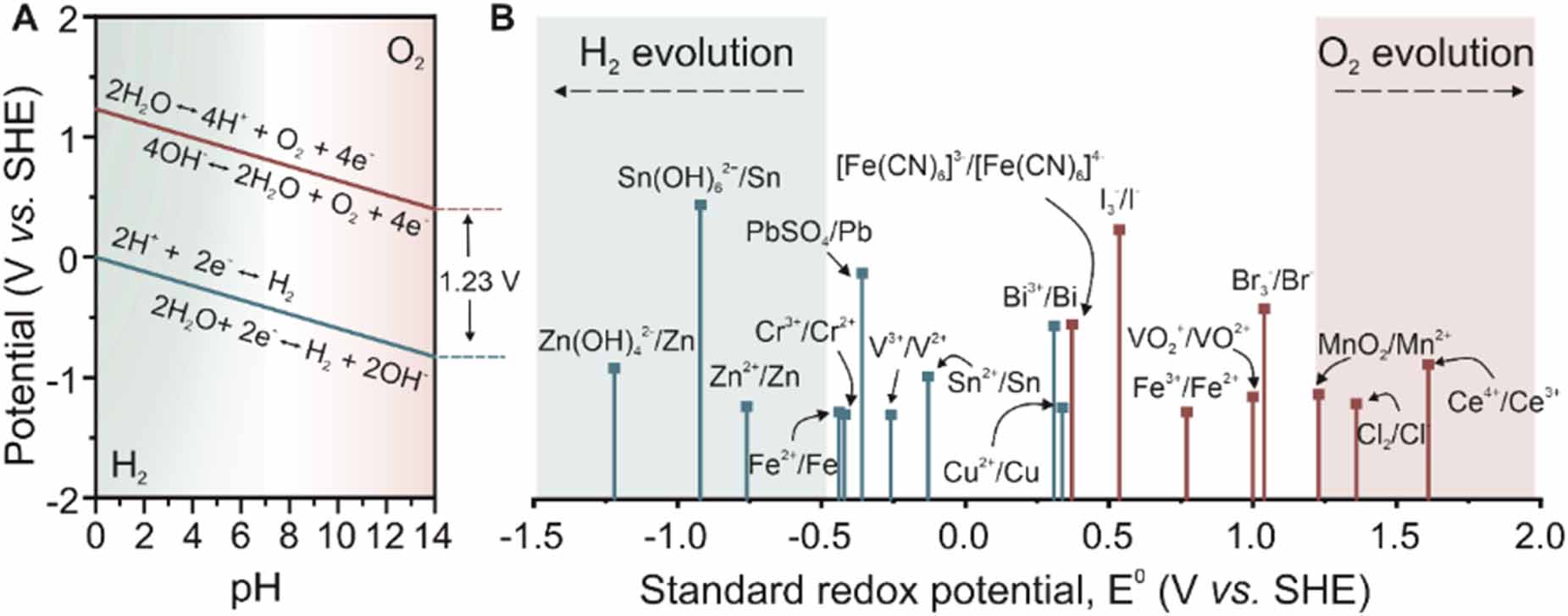
The electrochemical stability window of water is 1.23 V at 25 C, above which oxygen evolution reaction (OER) on the positive electrode and hydrogen evolution reaction (HER) on the negative electrode will occur. However, the actual water decomposition process usually requires an overpotential (
The potential difference between redox couples determines the operation voltage of the cell (figure 2(B)) [14]. As illustrated in figure 1(C), oxidized vanadium species and vanadium cations are separated by an ion-selective membrane, and the redox reactions are independent of each other. The overall voltage (1.26 V) is determined by redox potentials of VO2+/VO2+ (+1.00 V vs standard hydrogen electrode (SHE)) and V3+/V2+ (-0.26 V vs SHE) [15]. Besides, a mixture electrolyte would also be possible for specific redox reactions at different electrodes. For example, redox reactions for Zn2+ and Br- ions are able to individually occur at the anode and cathode in ZnBr2 solution (figure 1(G)) [16].
|
The redox potential of redox couples is also influenced by the concentration of the electrolyte following the Nernst equation: E=E0′+RTnFln[Ox][Re] where
|
|
Electrochemical reactions are heterogeneous with two successive processes: (a) the mass transport for the active species across the diffusion layer and (b) the kinetics for charge transfer across the electrolyte-electrode interface. Fast mass transport and electron-transfer kinetics allow for high energy efficiency and power density. The diffusion coefficient (
|
The results of peak currents obtained from a series of cyclic voltammetry (CV) measurements at various scan rates are brought into the Randles-Sevcik equation to derive the D value. In specific, the peak current shows a linear increase with the square root of the scan rate for a reversible system. The obtained slope is proportional to the D value. Nevertheless, the accuracy of the result may be impacted by capacitive effects [21]. Chronoamperometry measurements can yield the D value in a single experiment through the Cottrell equation [22-24]. An important parameter applied in the equation is the area of an electrode. The difference between the geometric area and the electrochemically active area would influence the results. By contrast, the definition of D value by LSV has a high accuracy due to the minimization of capacitive effects and natural conditions. On the other hand, the k0 value also can be yielded by the Nicolson method based on a series of CV measurements which are the same as the CV techniques carried out for deriving the D value [25]. Both D and k0 are essential parameters in any electrochemical process and have been discussed in detail in another focused review [26].
|
Vanadium (V) is an element with multiple valence states, among which V (+2, +3, +4 and +5) are typical redox-active species applied in redox flow batteries. The corresponding ions are V2+, V3+, VO2+ and VO2+ in sulfate solution. Redox reactions are: V3++e−↔V2+E0=−0.26 V vs SHEVO2++2H++e−↔V3++H2OE0=0.34 V vs SHEVO+2+2H++e−↔ VO2++H2OE0=1.00 V vs SHE.All redox-active cations can be generated reversely in a mixed electrolyte, circumventing the cross-contamination issue of decoupled electrolyte designs. The V3+/V2+ couple shows a negative standard redox potential of -0.26 V, thus acting as an anolyte. For the catholyte, the VO2+/VO2+ couple is employed due to its 1 V redox potential. The full-cell voltage reaches more than 1 V. The redox potential of VO2+/VO2+ changes at a rate of -59 mV per pH, while the potential of the V3+/V2+ redox reaction is pH-independent [
|
|
The solubility of VOSO4 (V4+ compound) and the stability of V5+ cations impose a limit on the working temperature range of -5 C40 C. More specific, 2 M VO2+ dissolved in 5 M H2SO4 precipitates at -5 C after only 18 h, and V2O5 precipitation is observed at above 40 C through the hydrolysis reaction of VO2+: 2VO+2+H2O ↔V2O5+2H+.
|
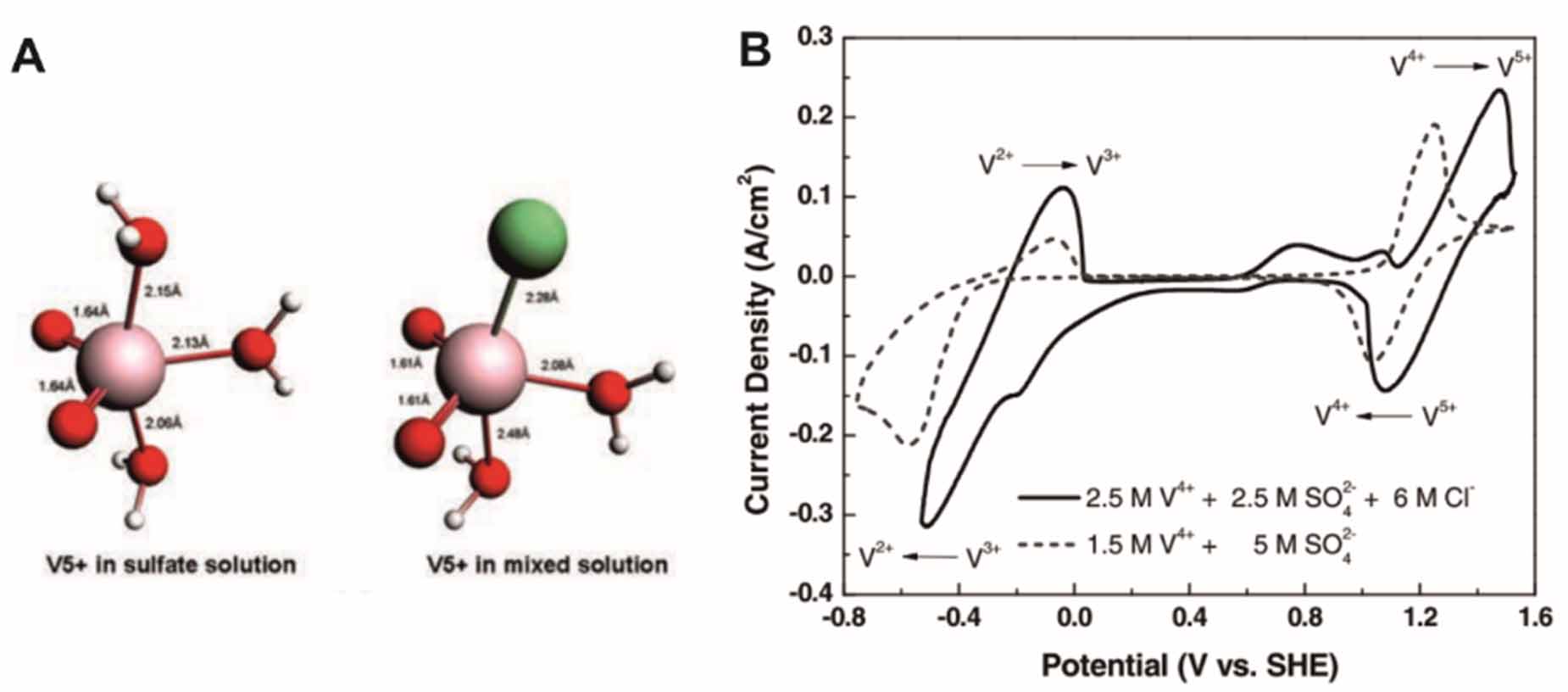
The stability of VO2+ cations increases at a higher concentration of sulfuric acid because excessive protons change the reaction quotient. A high concentration of H2SO4 increases the viscosity and therefore limits the mass transportation of active species. The employment of a mixed sulfate-chloride electrolyte is able to increase the solubility and enhance the stability of VO2+ in a wide range of operating temperatures [28, 29]. As a result, this system yields an approximately 70% increase in energy densities compared to the sulfuric-acid-based system. Li et al [29] reported a sulfate-chloride mixed solution (2.5 M H2SO4 and 6 M HCl) capable of dissolving 2.5 M vanadium cations. This battery has a wide temperature range of -5 C to 50 C. Such a temperature range expansion is attributed to the formation of stable VO2Cl(H2O)2 at elevated temperatures (>20 C) (figure 3(A)). Moreover, the electron transfer kinetics was improved, as evidenced by an increased peak current in CV curves of vanadium redox reaction in the sulfate-chloride mixed solution (figure 3(B)). Agarwal et al [30] elaborated that the charge transfer through the chloride bridge in HCl possesses higher polarizability than through surface-bound hydroxyl groups in H2SO4 and HCl/H2SO4, resulting in faster V3+/V2+ kinetics. However, HCl vapor at a high temperature and Cl2 evolution due to overcharging reduces the advantage of the fast reaction kinetics. Alternatively, catalysts, such as Bi [31, 32], Pt [33], and Ir [34], Mn3O4 [35, 36], MoO2 [37], GeO2 [38], and WO3 [39], and surface modifications by functional groups [40] (e.g. -NH2, -SO3H, and -C-OH, etc) are also effective in improving the reaction kinetics. It is worthy to notice that the affection of some oxygen-containing groups, however, reduces the kinetics of V3+/V2+ and VO2+/VO2+ [41].
Precipitation inhibitors can be used to stabilize VO2+ ions in sulfuric acid and thus increase the concentration [42, 43]. Skyllas-Kazacos et al [43] investigated the effects of phosphate additives, including ammonium phosphate ((NH4)3PO4), potassium phosphate (K3PO4), sodium hexametaphosphate (Na6[(PO3)6]), phosphoric acid (H3PO4), as well as the ammonium sulfates ((NH4)2SO4). The H3PO4 (1 wt.%) and (NH4)2SO4 (2 wt.%) combination prevent V5+ precipitating at a high temperature of 45 C thanks to the molecular interactions between VO2+ cations and NH4+/PO43- ions. More than that, (NH4)2SO4 can enhance the stability of V3+ in sulfuric acid at a low temperature of 5 C, offering a wide operating temperature range [44]. Other additives, such as polyacrylic acid [45] and trishydroxymethyl aminomethane [46, 47], also show proven efficacy in stabilizing the vanadium electrolytes.
Recent studies also focus on the fabrication of electrodes [48], application of novel membranes [49, 50], and the design of battery configurations. For instance, the modification of bipolar plates and flow field can effectively improve the overall performance [51, 52].
|
The low redox potential of the plating and stripping of Fe render the Fe2+/Fe couple available as the anode reaction: Fe2++e−↔Fe E0=−0.44 V vs SHE.
|
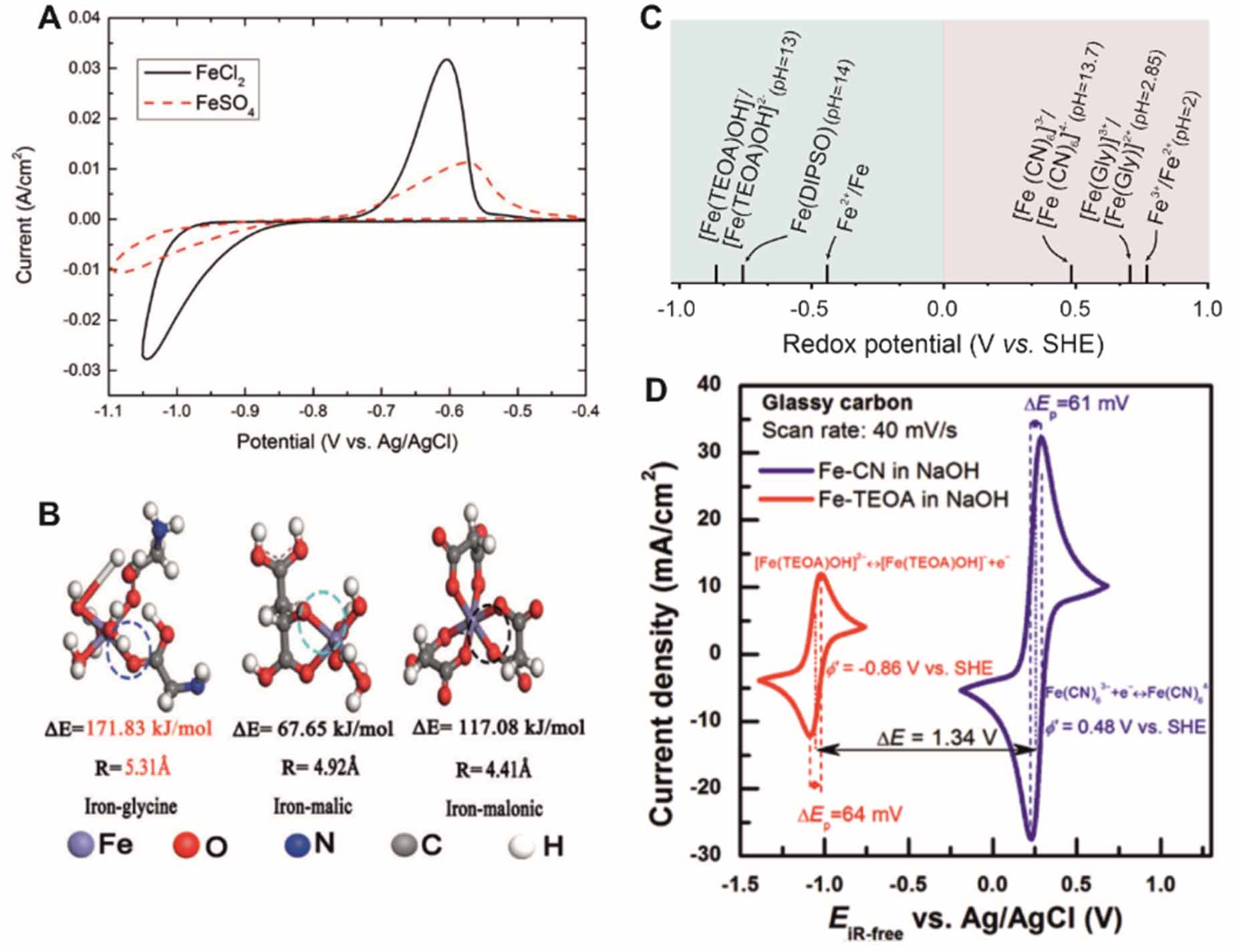
Apparently, the redox potential is 440 mV lower than for HER, suggesting that HER could cause coulombic losses during the plating and stripping of Fe [53]. Upon HER, pH may rise locally at the electrode surface or even in the bulk electrolyte, causing the precipitation of iron complexes. Buffering agents, ascorbic acid and citric acid for instance, prevent the rapid pH changes during the plating and stripping of iron [54, 55]. HER can be inhibited by increasing the electrolyte pH or limiting the mass transport of hydronium ions (H3O+). Increasing the pH of the electrolyte will shift the equilibrium potential for HER more negatively and slow down the mass transport. For example, Savinell et al [56] reported that the HER current decreased from 15 to 0.7 mA cm-2 at -0.8 V (vs Ag/AgCl) as the pH value increased from 1 to 3. However, if the pH increases beyond 3, Fe2+ will precipitate as hydroxides. Another approach to suppress HER is to remove potential absorption sites for protons by electrolyte additives, Cl- for instance (figure 4(A)) [56]. Depositing a thin metal layer (e.g. In, Cd) with a high overpotential of HER can also inhibit HER [55].
|
The Fe3+/Fe2+ couple is an excellent catholyte component:Fe3++e−↔Fe2+E0=0.77 V vs SHE.
|
The redox potential is pH-independent and has the standard rate constant (k0) of 1.2 10-4 cm s-1 [57]. Fe3+ ions are stable in a strong acidic solution but will hydrolyze and form Fe(OH)3 precipitations even at a low concentration of 10-6 M when the pH value exceeds 2 at 25 C [58]. One approach to prevent this issue is to link Fe3+/2+ ions with ligands. Ferricyanide ([Fe(CN)6]3-) and ferrocyanide ([Fe(CN)6]4-) are stable in a pH range extending to mild alkaline regime conditions due to the strong coordination of cyanide ions to the iron center. The standard potential of [Fe(CN)6]3-/[Fe(CN)6]4- reaction is 0.36 V vs SHE. As [Fe(CN)6]3- will decompose in a strong alkaline electrolyte, a neutral electrolyte shows improved stability for the [Fe(CN)6]3-/[Fe(CN)6]4- redox couples [59]. Besides, cations for [Fe(CN)6]3- and [Fe(CN)6]4- play an important role in the cell energy density because they would increase the solubility of [Fe(CN)6]3- and [Fe(CN)6]4-. For example, the solubility of (NH4)4[Fe(CN)6] is 1.6 M in the water while it is only 0.56 M for Na4[Fe(CN)6] [60].
Ligands alleviate ion crossover due to the large size of the complexes. A large ligand, such as glycine, offers a large complex with a radius of 5.31 (figure 4(B)), suppressing the hydrolysis of Fe3+/Fe2+ due to the high binding energy and reducing the crossover rate [24]. Chelation of ligands reduces the activation energy of electron transfer of Fe3+/Fe2+, thus improving the reaction kinetics [61-64]. Moreover, the variation of the electron cloud of iron by complexing with different ligands leads to a range of reduction potentials (figure 4(C)), providing diverse matchups for the iron-based redox electrolytes. For instance, a couple of [Fe(CN)6]3-/[Fe(CN)6]4- as catholyte and [Fe(TEOA)OH]-/[Fe(TEOA)OH]2- (-0.86 V vs SHE) as anolyte shows a voltage of 1.34 V in an alkaline electrolyte (figure 4(D)) [23].
|
The first aqueous redox flow battery was invented and launched by NASA in the 1970s, in which the Fe3+/Fe2+ couple as the catholyte and the Cr3+/Cr2+ pair as the anolyte. The redox reaction of Cr3+/Cr2+ is expressed by the following equation [
|
As the redox potential is more than 400 mV lower than that for HER, such a redox reaction often exhibits a low coulombic efficiency due to the parasitic HER process. Additionally, the sluggish kinetics of Cr3+/Cr2+ is relatively low, which requires the incorporation of catalysts to facilitate the redox reaction [66].
Iodide (I-) shows high solubility in aqueous and non-aqueous media. The oxidation of iodide to iodine (I2) is followed by the formation of triiodide (I3-) through I- complexation [67, 68]:
|
2I−−2e−↔I2(aq)E0=0.621 V vs SHEI2+I−→ I−3.
|
|
Accordingly, excessive iodide ions are used as a complexing agent to stabilize the free iodine, thus giving an overall reaction as follows [
|
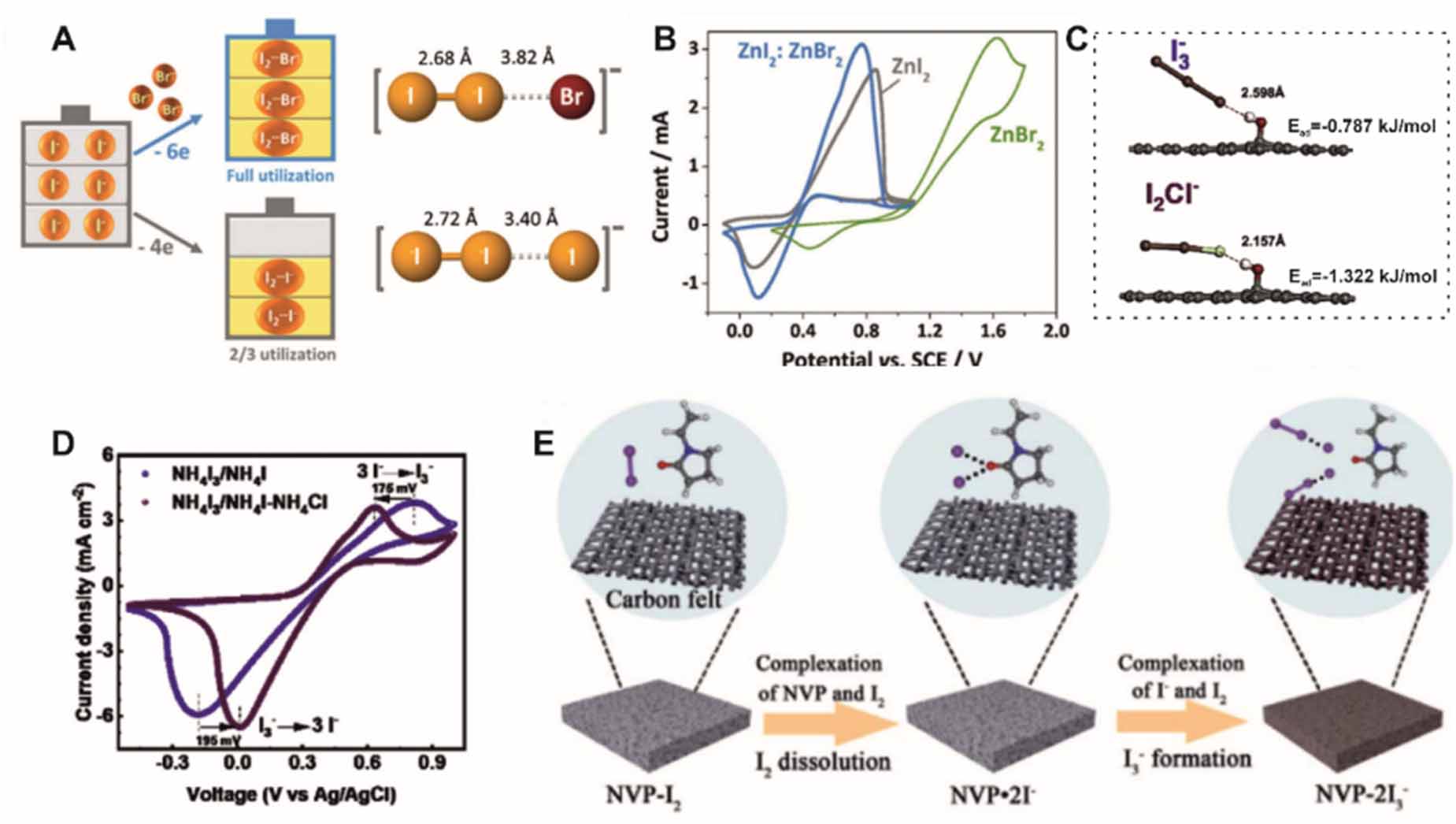
The I3-/I- couple, instead of the redox couple of I2/I-, is used in catholyte [71]. However, the low utilization of I- limits its volumetric capacity and therefore energy density. Complexed with other halogen elements, such as bromide ions, more I- ions were involved in the energy storage because I2 can be stabilized by complexing with bromide ions (I2Br-, figure 5(A)) [72]. Meanwhile, the generation of corrosive bromine was excluded during redox reactions (figure 5(B)). As a result, a high energy density of 202 Wh l-1 based on the catholyte volume was achieved in a Zn/iodine-bromine (I2Br-) flow battery. Iodine-chloride (I2Cl-) was also capable of increasing the cell capacity [73]. I2Cl- anions not only improves the reaction reversibility (figure 5(C)), but also shows high adsorption energy to the OH-functionalized graphite felt as evidenced by the reducing X-H bond length (figure 5(D)). In practice, I3- can be oxidized to solid-state I2 by overcharging, and the accumulated I2 forms a film on the electrode, blocking the pore of electrodes and the pumps or pipelines [74]. Carbonyl groups in N-vinylpyrrolidone (NVP) can break I-I bonds in I2, forming NVP-2I3- (figure 5(E)) [75]. Therefore, NVP is an excellent additive to prevent the accumulation of I2.
Despite all these downsides brought by solid I2, the liquid-solid conversion of I- to I2 can realize an almost 100% utilization of I-, offering a high energy density of 205 Wh l-1 approaching its theoretical energy density [76]. In this case, the pump and pipeline blockage caused by solid I2 can be bypassed by avoiding electrolyte circulation.
Likewise, many bromide salts are highly soluble in water. A high redox potential of the Br2/Br- couple (1.08 V vs SHE) contributes to a high cell voltage. Similar to iodide oxidation, bromine molecules can combine with bromide ions to generate tribromide ions [77]:
|
2Br−−2e−↔ Br2E0=1.08 V vs SHE Br2+ Br−→ Br−3.
|
As a result, the overall reaction is:
|
3Br−−2e−↔ Br−3.
|
Several redox couples have been employed to pair with Br2/Br- cathode to generate aqueous redox flow batteries, such as zinc-bromine, vanadium-bromine, hydrogen-bromine and quinone-bromine cells. However, the high toxicity (0.1 ppm of OSHA Permissible Exposure Limit) and high vapor pressure (28.8 kPa at 25 C) should be considered in practical use. Complexing with a ligand is also an effective method to mitigate the toxicity of Br2.
Sulfur and polysulfides show rich and complicated chemistry. Transformation of polysulfides with different chain lengths show solid-liquid, liquid-liquid, and even the solid-solid conversion. Such diverse reactions offer broad opportunities to design high-capacity batteries. Unlike in an organic solvent, in which long-chain polysulfides (e.g. Li2Sn, n 4) can be dissolved, short-chain polysulfides (including Li2Sn, Na2Sn, and K2Sn, 1 n 4) are highly soluble in water. The negative redox potential of the transition between various polysulfides enables their application of anolyte in flow batteries [78, 79]:
|
S2−4+2e−↔2S2−2E0=−0.51V vs SHES2−2+2e−↔2S2−E0=−0.51V vs SHE.
|
The former reaction has a lower capacity of 418 mAh g-1 while the latter offers a superior capacity of 837 mAh g-1. In addition, a shorter chain shows improved kinetics. The rate of mass transport is lower than the charge transfer process without strong convection (e.g. stirring). Therefore, external stirring or circulation of electrolyte and the regulation of electrolyte concentration have been frequently used to enhance the mass transport to alleviate the concentration polarization. Plenty of approaches have been employed to boost the performance of S-based aqueous batteries. A detailed review on polysulfide redox couples has recently been given by Chao et al [80].
|
Manganese (Mn) has diverse valence states (i.e. 0, +2, +3, +4, +6 and +7) offering rich redox chemistry. The charge storage of Mn-based cathodes often relies on solid-state conversion and cation (Na+, Zn2+ and H+) intercalation. The mass-loading of active electrode materials predetermines the capacity. Recently, the deposition-dissolution reaction of the MnO2/Mn2+ redox couple was proposed by Cui
|
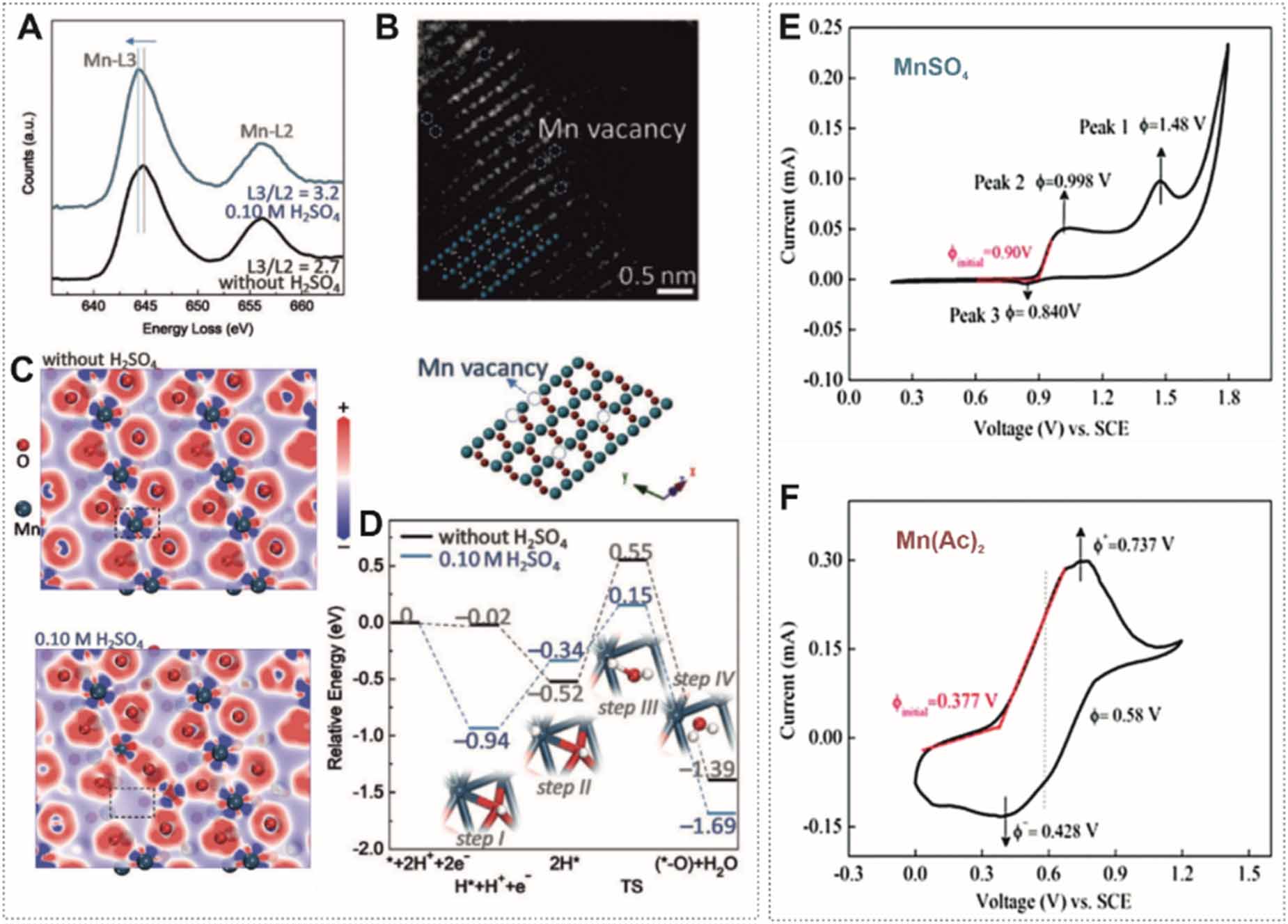
The two-electron transfer allows for a high theoretical capacity of about 616 mAh g-1 based on the mass of MnO2. Two mechanisms were proposed: (a) hydrolysis in neutral electrolyte and (b) disproportionation in acidic electrolyte. Both mechanisms start with the oxidation of Mn2+ forming Mn3+ through a one-electron reaction. Subsequently, Mn3+ are hydrolyzed and precipitate as MnOOH on the surface electrode, which is further oxidized to MnO2 in a neutral solution or undergoes a disproportionation reaction producing soluble Mn2+ and Mn4+ ions hydrolyzed to MnO2 in an acidic solution. As Mn3+ is stable in the acidic solution, the mixed oxidation state of Mn3+ and Mn4+ are often found in the electrodeposited manganese oxide. Qiao et al [82] compared the electrodeposited MnO2 from a solution (1 M ZnSO4 and 1 M MnSO4) with and without adding 0.1 M H2SO4. The mixed oxidation state of Mn4+/Mn3+ in the electrodeposited MnOx was derived from x-ray photoelectron spectra and Mn-L2,3 electron energy loss spectra (EELS) (figure 6(A)). Mn vacancies were directly observed in the MnO2 obtained from the mildly acidic solution (figure 6(B)). The increased surface energy density from Mn vacancies reduces the energy barrier and accelerates the dissolution of MnO2 (figures 6(C) and (D)).
|
However, the disproportionation reaction of Mn3+ species reduces the energy density because fewer electrons can be stored (figure
|
In this case, Mn(Ac)2 is added to the electrolyte. The reaction exhibits high reversibility. The oxidation potential of MnO2 in the Mn(Ac)2 system is 0.53 V, lower than in MnSO4 (figure 5(F)), which is ascribed to the lower Gibbs free energy of HAc that requires less energy to trigger the oxidization.
Zinc (Zn) metals have advantages of low cost, high capacity, and inherent stabilities in air and aqueous solutions. The plating-stripping process of Zn features different couples with a variation in pH. The detailed reactions in different media are as follows:
|
Zn2++2e−↔Zn E0=−0.76V vs SHEZn(OH)2−4+2e−↔Zn+4OH−E0=−1.22V vs SHE.
|
The redox potential of Zn/Zn2+ is -0.76 V (vs SHE) in neutral or mild acidic electrolytes. However, Zn metal suffers from severe dendrite growth and accompanied HER process. In alkaline electrolytes, the Zn(OH)42-/Zn couple exhibits fast kinetics and a much lower redox potential [84]. Nevertheless, the Zn reversibility still decreases due to the formation of byproducts.
|
Tin (Sn) can be reversibly stripped and plated at a potential of -0.13 V (vs SHE):Sn2++2e−↔SnE0=−0.13 V vs SHE.
|
The high diffusivity of Sn2+ ions in chloride acid (1.67 10-5 cm2 s-1) and fast reaction kinetic constant (2.02 10-4 cm s-1) guarantee good performance as a redox-active electrolyte [22]. Moreover, in an alkaline electrolyte, the Sn(OH)62-/Sn electrode shows a smooth and dendrite-free morphology due to the intrinsic low-surface-energy anisotropy that facilitates the isotropic crystal growth of the Sn metal. As a result, the Sn(OH)62-/Sn anolyte offers high reversibility of up to 500 stable cycles (more than two months) [85].
Organic redox-active species often have tunable potentials by designing functional groups. For instance, the reduction potential of quinones decreases because electron-donating groups (e.g. -OH and -NH2) increase molecular orbital energies. On the contrary, electron-withdrawing substituents (e.g. -SO3H) reduce the molecular orbital energies, thus raising the reduction potential [86-89]. The pH variation also influences the redox potential of quinone-based redox couples. The tunable redox potential allows for the design of all quinone-based battery system [87, 90]. Similar phenomenon can be found on 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) derivatives. Redox-active nitroxyl radicals show tunable potentials with various functional groups [91]. However, TEMPO derivatives are generally employed in the catholyte due to their relatively positive potential [92].
The functional groups also play an important role in manipulating the solubility of organic redox species. For instance, methyl viologen (4,4-dimethyl bipyridinium dichloride, MVi2+) has a high solubility of 3 M in water. It undergoes a two single-electron reduction at negative potentials (-0.45 V and -0.76 V vs SHE). The first reduction shows high reversibility, while the second one becomes quasi-reversible due to the low solubility of the corresponding reduction product, which is a common dilemma for viologen-based redox couples. Many efforts have been carried out on enhancing the solubility of the final reduction product by substituting with a hydrophilic functional group (e.g. hydrophilic ammonium or sulfonate functional group) to enable high reversibility of the second reduction [87, 90, 93-95]. In addition, Viologens and TEMPO derivatives show high stability in the neutral electrolytes and therefore a broad potential window [96, 97].
Redox-flow batteries storing the redox-active electrolytes in external tanks (figure 7(A)) represent a straightforward design to unleash the energy storage ability of electrolytes. Catholytes and anolytes are stored in independent reservoirs and circulated with an exterior pump. The continuous supply of electrolytes via circulation can reduce the concentration polarization by refilling redox-active species timely after they are consumed to store energy. Carbon-based materials (e.g. carbon felts or carbon papers) are widely used as electrodes because they provide abundant active sites for redox reactions. Further modification on the carbon surface and electrocatalysts are used to improve energy efficiency. A membrane, known as an ion-selective membrane, is placed between the catholyte and anolyte to prevent the ion crossover.
A simplified design was created by using an electrolyte containing redox-active species for cathodic and anodic reactions. For instance, the ZnBr2 electrolyte offers Zn2+/Zn and Br-/Br redox couples. The ion transportation of anions should be fast enough to warrant redox reactions at the cathode without the external circulation force. A zinc-iodine (Zn-I) single flow battery shows a high energy density of 205 Wh l-1 (theoretical energy density is about 240 Wh l-1 based on the electrolyte volume). The combination of a highly ionic conductive polyolefin separator and highly cation-selective Nafion thin membrane results in high energy efficiency.
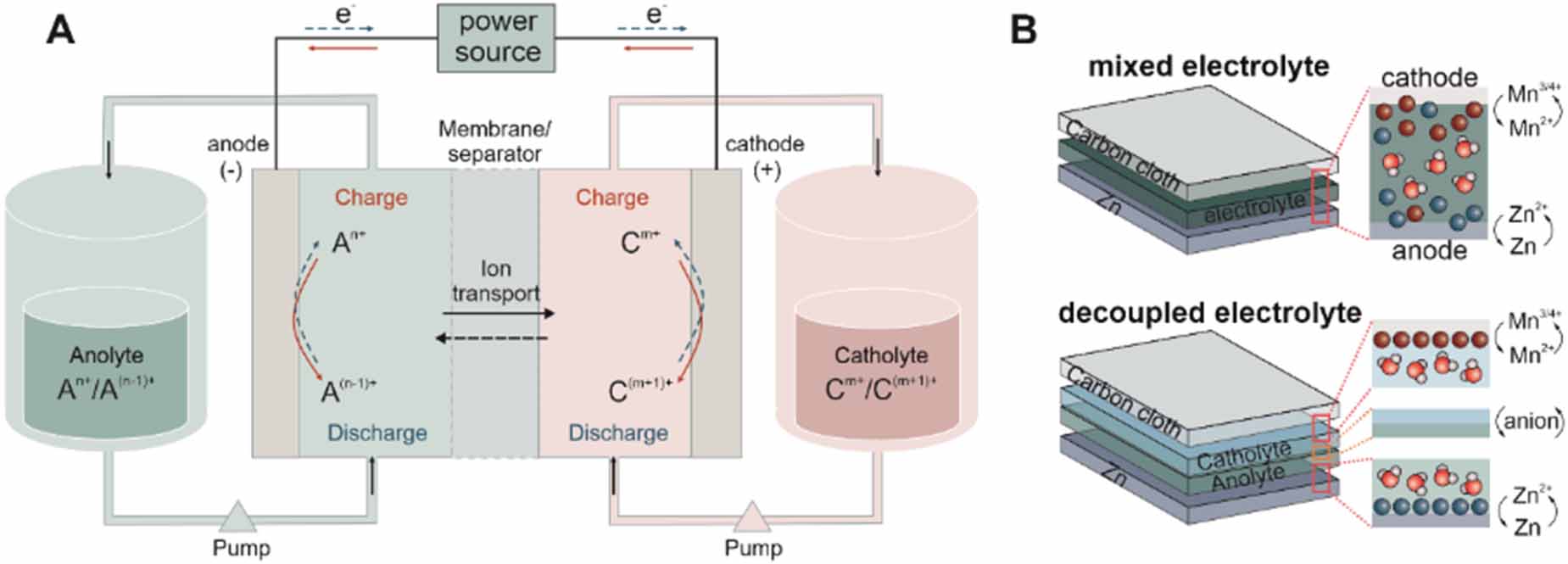
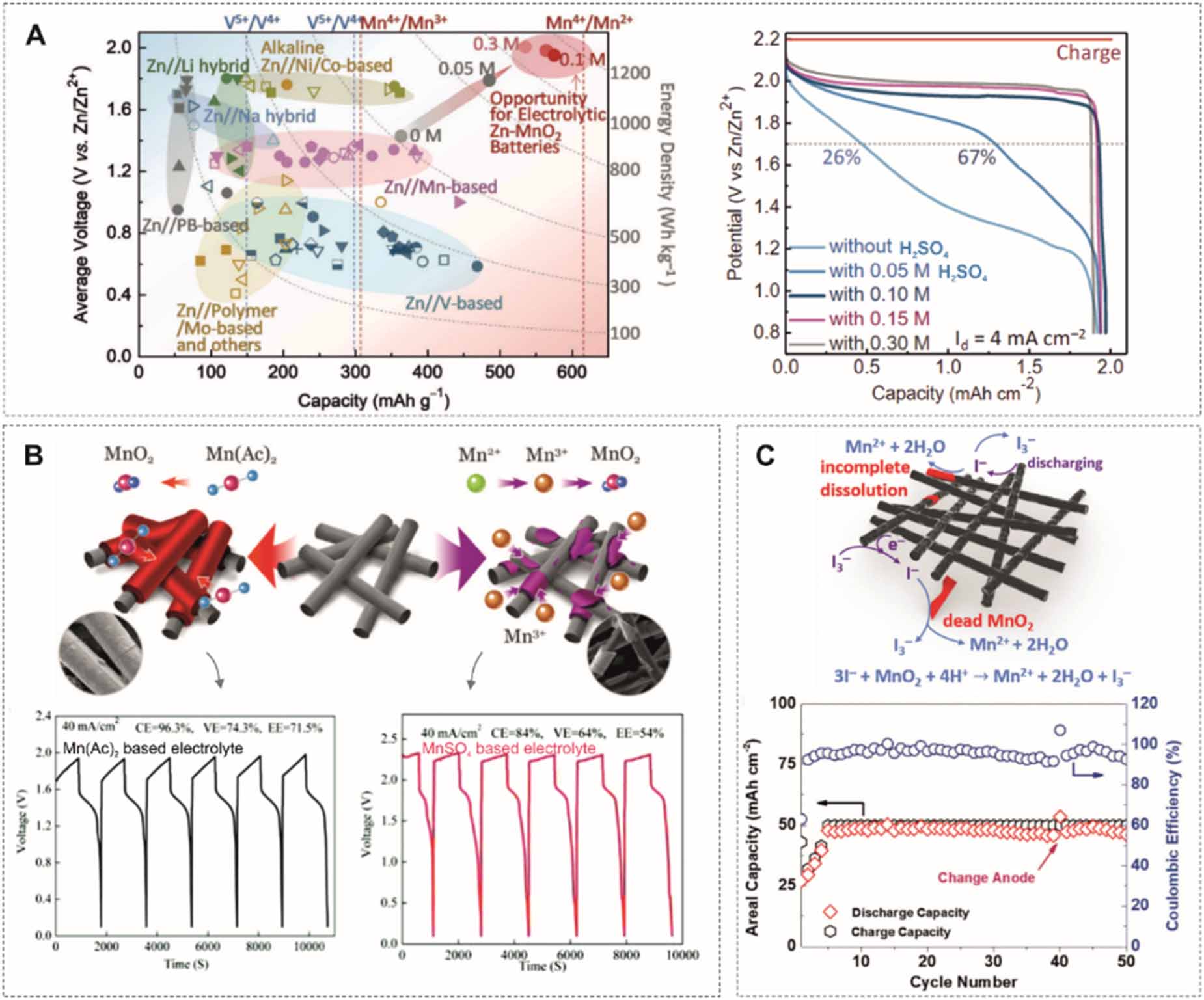
When used in portable devices, it is impossible to circulate the redox-active electrolytes from an external electrolyte tank. As a result, cell structures follow the conventional design with a sandwich structure (figure 7(B)). Mn2+/Mn4+ and Zn/Zn2+ redox couples were first investigated to unleash the energy storage in the electrolyte because they share the same device structure as alkaline or neutral Zn-MnO2 batteries. Qiao et al [82] applied MnO2/Mn2+ conversion in an acidic electrolyte (figure 8(A)). The battery exhibits a high discharge voltage (1.95 V). With a high capacity of 571 mAh g-1, the battery delivers a high energy density of 409 Wh kg-1 based on the total mass of active materials. Despite the fast kinetics of MnO2/Mn2+ conversion, the side reaction of Mn3+ disproportionation would inhibit the conversion efficiency between MnO2 and Mn2+. In general, the oxidation states of Mn3+ and Mn4+ are observed in the deposited MnO2. To improve the energy efficiency, Li et al replaced MnSO4 by Mn(Ac)2 (figure 8(B)), which enables the direct conversion from Mn2+ to MnO2 by coordinating Ac- anions [83]. Although the battery exhibits a lower discharge voltage plateau (1.5 V) than for the MnSO4 electrolyte (1.8 V), higher efficiencies, including coulombic efficiency, voltage and energy efficiency, were achieved. The battery worked well with an area capacity of 20 mAh cm-2, which was previously limited to 10 Ma h cm-2. Lu et al introduced an iodide (I3-) redox mediator into the electrolyte to reduce the dead’ and undissolved MnO2 (figure 8(C)), increasing the charge capacity up to 50 mAh cm-2 [99]. Likewise, Chen et al [100] employed Br3-/Br- mediator to improve the dissolution of the over deposited MnO2 and enhance the reversibility of the cathodic reaction. The acidic electrolyte facilitates the Mn2+/Mn4+ conversion but brings a high risk of Zn corrosion. Zhu et al [98] decoupled the electrolyte into acidic catholyte and mild anolyte in the gel state, resulting in more than two times higher capacity and rate capability.
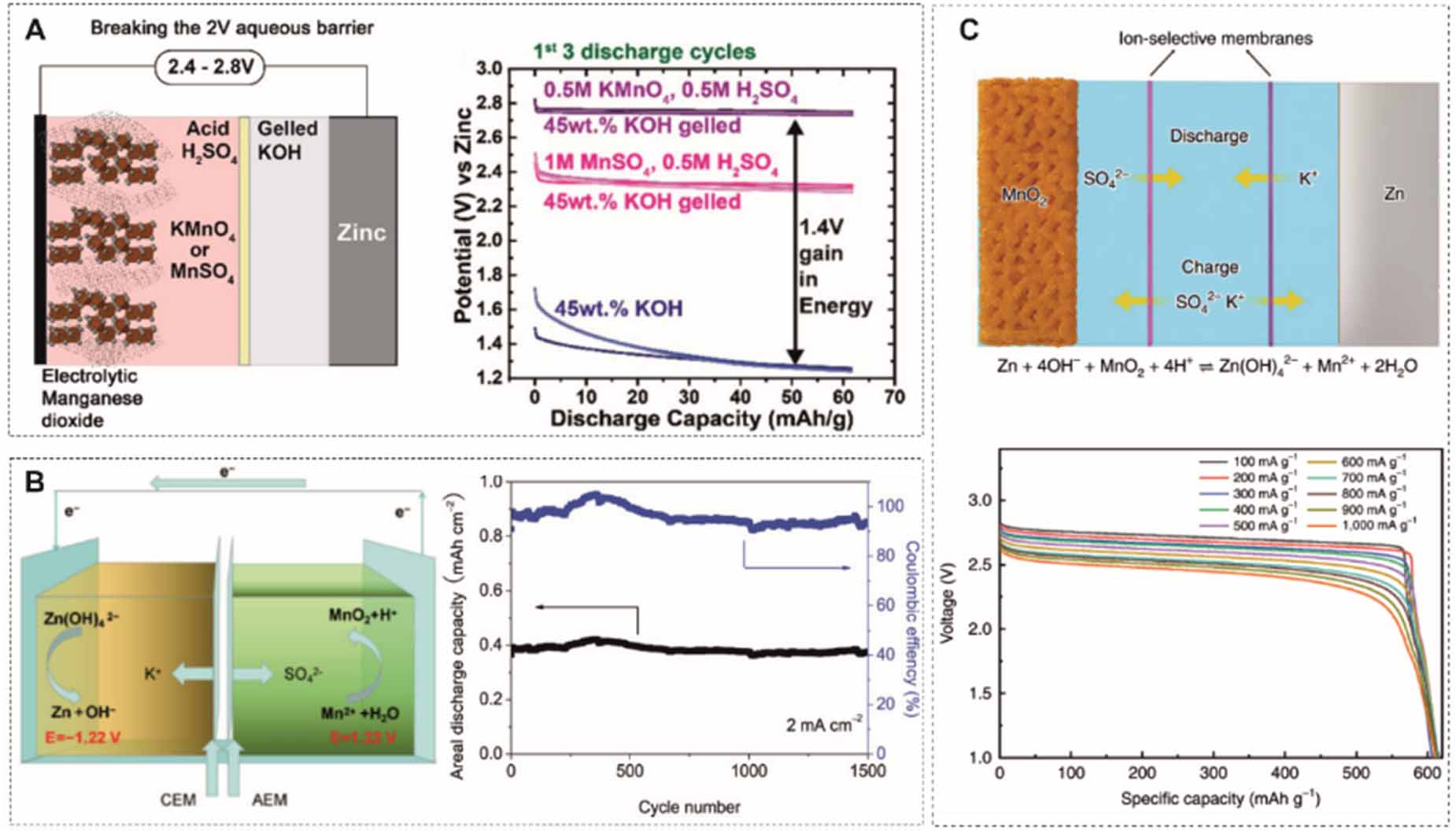
Based on the decoupled design, the anolyte can be changed to an alkaline solution to further reduce the redox potential of the anode. The Zn(OH)42-/Zn redox couple in the alkaline media has a potential of -1.22 V vs SHE, which is about 0.46 V lower than for mild and acidic electrolytes. Banerjee et al [101] came up with a design based on an acidic-alkaline dual electrolyte, which exhibits a 2.45 V open circuit voltage. The MnO2 pre-coated carbon felt is immersed in an acidic liquid electrolyte, and the Zn anode is surrounded by a polymerized gelled alkaline electrolyte (figure 9(A)). The decoupled electrolyte removes the limit of water splitting at 1.23 V and therefore expands the electrochemical stability window of the electrolyte. Liu et al [102] presented a similar design by using the liquid acid-alkaline electrolyte. The catholyte contains 1 M MnSO4 and 0.5 M H2SO4, and the anolyte consists of 0.1 M Zn(Ac)2 and 2.4 M KOH (figure 9(B)). To prevent neutralization, two solutions are separated by a bipolar membrane, anion exchange membrane and cation exchange membrane. The battery shows a high CE of 98.4% and cycling stability of 97.5% retention over 1500 cycles. As the potential of MnO2/Mn2+ conversion is pH-dependent, the cell voltage can be further raised to 2.65 V by employing a strong acidic catholyte with 3 M H2SO4 (figure 9(C)) [103]. In particular, the battery has a discharge plateau as high as 2.71 V at a current density of 100 mA g-1 with a specific capacity of 616 mAh g-1, achieving 100% of the theoretical capacity of the two-electron transfer process of Mn4+/Mn2+. Both high working voltage and specific capacity enable a high energy density of 1621.7 Wh kg-1 based on the mass of MnO2. Moreover, the battery shows a superior cycling stability of 200 h (116 deep cycles) due to the enhanced dissolution of MnO2 during discharge in strong acid. The adsorbed hydrogen ions on the MnO2 surface facilitate the formation of oxygen vacancies and lead to Mn atoms being exposed on the surface. It is also possible to introduce a catalyst, such as Ni, to catalyze MnO2/Mn2+ conversion [104].
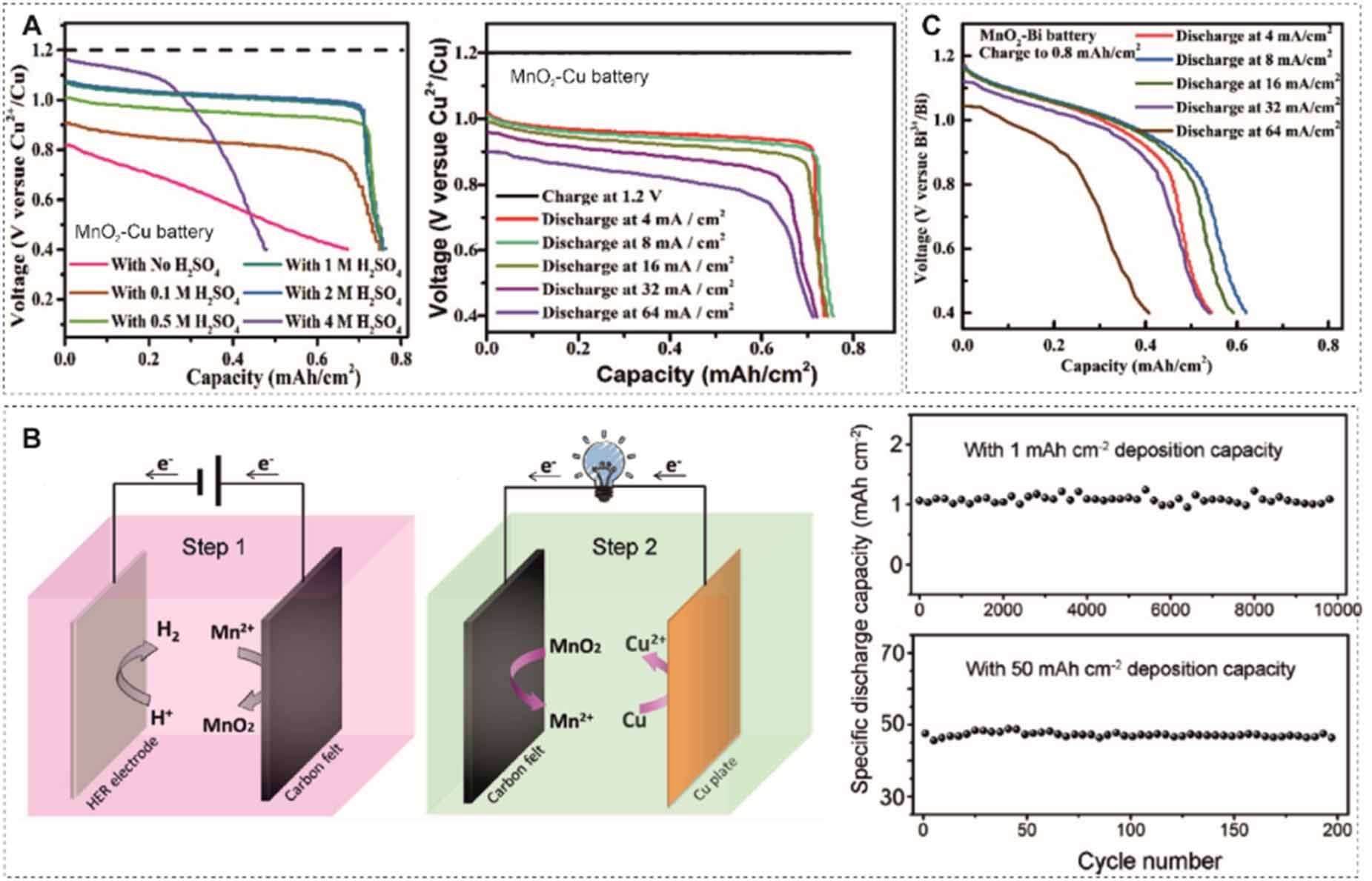
Other metal-based anodes (e.g. Cu, Bi, Pb) are also paired with MnO2/Mn2+. For instance, MnO2-Cu and MnO2-Bi batteries were proposed by Zhi et al [105]. The MnO2-Cu battery exhibits an average discharge plateau at 0.95 V in an acidic electrolyte (figure 10(A)). It shows a stable rate capability with only a 6.6% decrement as the discharge current density increases from 4 to 64 mA cm-2 and a lifetime of 2000 cycles under a charge capacity of 0.8 mAh cm-2 at 16 mA cm-2. During long-term cycling the coulombic efficiency range fluctuates between 123.8% and 84.8%, which might be associated with the insertion of ions (H+ and Cu2+) into undissolved MnO2. Xia et al [106] prolonged the lifetime of a MnO2-Cu battery to 10 000 cycles under 1 mAh cm-2 capacity at 10 mA cm-2 without capacity decay. It used a high concentration of MnSO4 and CuSO4, up to 0.8 M. The cell capacity can reach up to 50 mAh cm-2 with a coulombic efficiency of 96% and a high energy efficiency beyond 70% (figure 10(B)). The energy density attains 40.8 Wh l-1. The MnO2-Bi battery displays a stable rate capability (0.4 mAh cm-2 at 64 mA cm-2 when charged to 0.8 mAh cm-2) in an acidic electrolyte but with a coulombic efficiency lower than 80% due to the uncomplete dissolution of MnO2 and the quasi-reversible plating and stripping process of the Bi metal (figure 10(C)) [105].
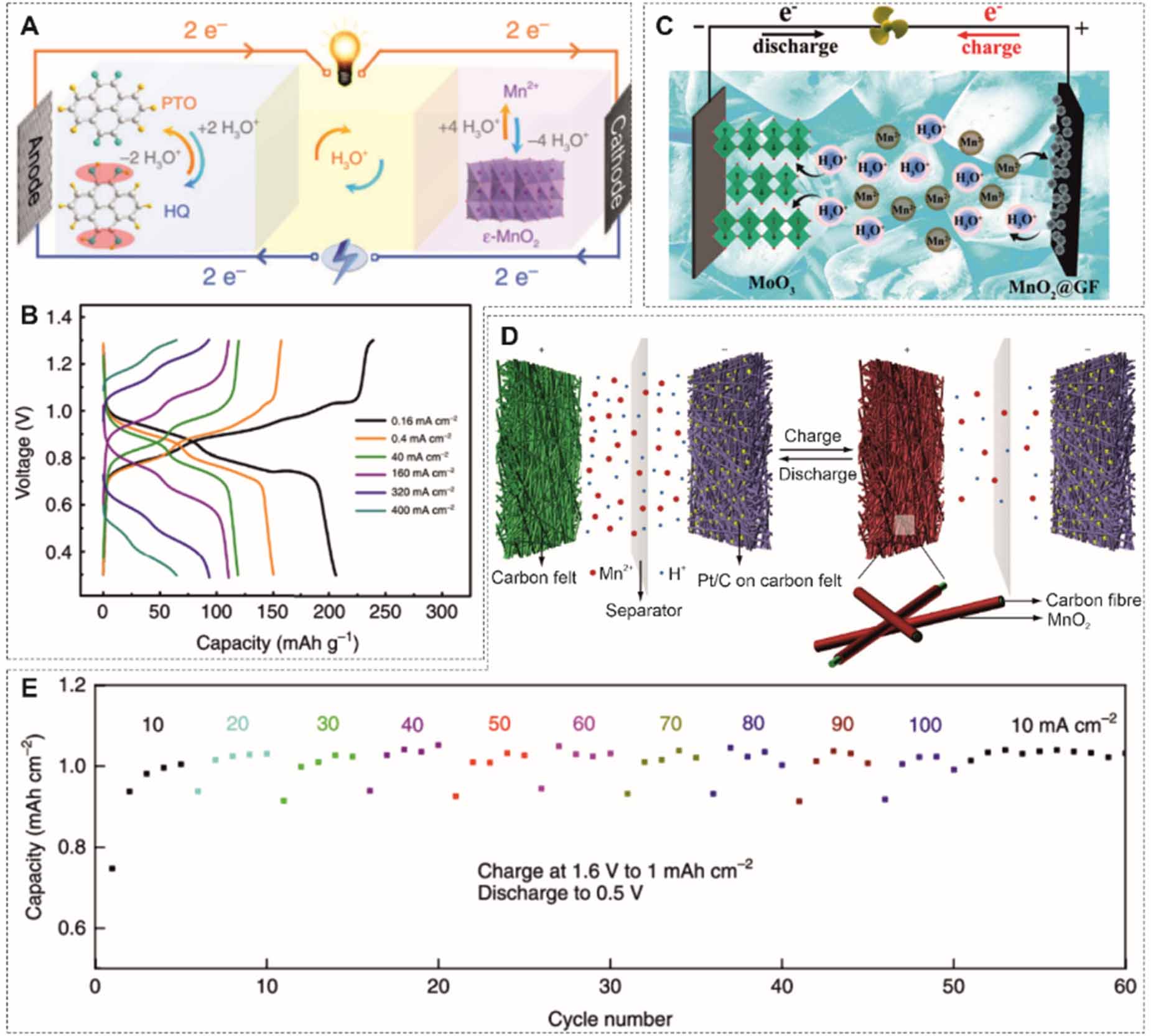
Besides metallic anodes, proton-coupled redox pairs can also be used as active species for the anode. Wang et al proposed a hydronium-ion battery, where pyrene-4,5,9,10-tetraone (PTO) was used as the anode [107]. The formed hydronium ions (H3O+) shuttle between electrodes, initiating a quinone/hydroquinone redox reaction at the anode and MnO2/Mn2+ conversion reaction at the cathode. A high power density of 30.8 kW kg-1 based on the total mass of PTO and deposited MnO2 is attainable due to the good diffusivity of hydronium ions (figures 11(A) and (B)). Moreover, the electrolyte still shows a high conductivity in a frozen state, enabling the operation of the battery from -40 C to -70 C. MoO3 also hosts H3O+ ions, which exhibits a power density of 66.6 kW kg-1 (figure 11(C)) [108]. Hydrogen can be directly used as the anode. Cui et al [81] proposed a Mn-H battery, where a Pt/C coated carbon felt was employed as the catalyst and conductive materials for H2 evolution. The 1 M MnSO4 electrolyte was optimized by adding 0.05 M H2SO4. The Mn-H battery exhibits a discharge voltage of 1.3 V, a good capacity of nearly 1 mAh cm-2, reaching almost 100% coulombic efficiency at 100 mA cm-2 (36 s of discharge), and a lifetime of more than 10 000 cycles without capacity decay (figures 11(D) and (E)).
The energy storage capacity of the electrolyte has shown its promise to further increase the energy density of batteries. However, such designs are mainly employed at the grid-scale and are rarely used in portable or microdevices. In order to fabricate excellent portable aqueous batteries based on such designs, both the working mechanisms and the technical construction are indispensable.
First of all, the working mechanisms are instructive roles to design energy storage devices with high electrochemical performance. However, the working mechanisms of the aforementioned redox-active electrolytes in the non-flow designs are still not fully uncovered. For example, protons are important for the reversibility of the Mn2+/MnO2 couple. Nevertheless, Mn3+ disproportionation reactions often occur with a high proton concentration, impairing the coulombic efficiency. A Zn-rich composite layer was found on the surface of MnO2 during the charging process, but it disappeared by discharging [109]. However, the exact crystal structure and function in energy storage process are still unknown. Thus, in-situ investigations, especially those focusing on the interface, would be helpful to reveal the reactions of the redox-active species in the electrolyte. Without a precise working mechanism, it is hard to develop effective ways to simultaneously improve the reversibility and efficiency of charge storage.
In addition to the working mechanism, the compatibility of materials with device fabrication procedures is also critical. Carbon felt has been frequently used as the current collector due to its high electrical conductivity. The porous structure provides abundant electrochemically active sites and reduces the areal current density, and the 3D matrix can accommodate high mass loading of MnO2 thus approaching a high charge capacity. However, if shrink the scale of the battery to a millimeter or even a micrometer, the carbon felt would be not available. The graphene or metal materials through physical vapor deposition or other deposition techniques should be considered. The deposited membranes are generally not able to offer such a 3D matrix unless with a design of 3D structures. It would also be a good approach to fabricate a planar thin film with a large footprint to introduce more redox reaction sites and shrink the footprint to form a Swiss-roll configuration through rolled-up nanotechnology. In addition, the accumulation and dissolution of redox products that introduce repeated and significant stress variation also impose a challenge in the mechanical stability of current collectors, especially for microscale devices that contain a thin current collector. The selection of the counter electrode is essential to the overall electrochemical performance. For instance, the dendrite formation and HER are two challenges using Zn as the anode. The optimized electrolyte for independent half-cells is not always applicable for a full cell. The addition of protons in the MnO2/Mn2+ deposition/dissolution system increases the reversibility; however, the high acidity accelerates the corrosion of Zn anode.
Besides, exploring a suitable solid-state electrolyte is an essential step toward practical use. Unlike common solid-state electrolytes that focus primarily on ionic conductivity, redox-active solid-state electrolytes also need to guarantee the redox activity of the ions. More importantly, the solid-solid interface between the electrolyte and electrode (current collector) after accumulating redox products needs to maintain the reactivity. The selection of packaging materials with high stability is often overlooked but important for the lifetime of actual devices.
Apart from the mechanism and battery fabrication, the operation protocols need to be different with different chemistry. The charging process determines the capacity of the battery using the redox-active electrolyte. With the increase in charge capacity, the current collector will be coated with a thicker material layer. The poor electrical conductivity of deposits would impair the reversibility. Accumulation of inactive deposits will eventually delaminate from the current collector, causing unwanted effects, such as short-cuts. Therefore, each electrolyte with redox activity should have its own optimal charging and discharging procedure and target specific application scenarios. It is a promising energy storage system by triggering and unleashing the energy storage ability of active species in electrolytes and it still needs more effort to put in, facilitating its development from grid-scale to a portable application or even a millimeter/micrometer scale.
M Zhu acknowledges the support by the German Research Foundation DFG (ZH 989/2-1). O G Schmidt acknowledges financial support by the Leibniz Program of the German Research Foundation (SCHM 1298/26-1). H Tang, Z Qu, W Zhang and H Zhang acknowledge the support and funding from China Scholarship Council (CSC).
Author to whom any correspondence should be addressed.
| [1] |
Gr T M 2018 Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage Energy Environ. Sci. 11 2696-767 DOI: 10.1039/C8EE01419A
|
| [2] |
Park M, Ryu J, Wang W, Cho J 2017 Material design and engineering of next-generation flow-battery technologies Nat. Rev. Mater. 2 16080 DOI: 10.1038/natrevmats.2016.80
|
| [3] |
Faisal M, et al 2018 Review of energy storage system technologies in microgrid applications: issues and challenges IEEE Access 6 35143-64 DOI: 10.1109/ACCESS.2018.2841407
|
| [4] |
Pal B, Yang S, Ramesh S, Thangadurai V, Jose R 2019 Electrolyte selection for supercapacitive devices: a critical review Nanoscale Adv. 1 3807-35 DOI: 10.1039/C9NA00374F
|
| [5] |
Lee J, et al 2019 Redox-electrolytes for non-flow electrochemical energy storage: a critical review and best practice Prog. Mater. Sci. 101 46-89 DOI: 10.1016/j.pmatsci.2018.10.005
|
| [6] |
Borenstein A, et al 2017 Carbon-based composite materials for supercapacitor electrodes: a review J. Mater. Chem. A 5 12653-72 DOI: 10.1039/C7TA00863E
|
| [7] |
Simon P, Gogotsi Y 2020 Perspectives for electrochemical capacitors and related devices Nat. Mater. 19 1151-63 DOI: 10.1038/s41563-020-0747-z
|
| [8] |
Choi C, et al 2020 Achieving high energy density and high power density with pseudocapacitive materials Nat. Rev. Mater. 5 5-19 DOI: 10.1038/s41578-019-0142-z
|
| [9] |
Kumar K S, Choudhary N, Jung Y, Thomas J 2018 Recent advances in two-dimensional nanomaterials for supercapacitor electrode applications ACS Energy Lett. 3 482-95 DOI: 10.1021/acsenergylett.7b01169
|
| [10] |
Leung P, et al 2012 Progress in redox flow batteries, remaining challenges and their applications in energy storage RSC Adv. 2 10125-56 DOI: 10.1039/c2ra21342g
|
| [11] |
Zhang J, et al 2018 An all-aqueous redox flow battery with unprecedented energy density Energy Environ. Sci. 11 2010-5 DOI: 10.1039/C8EE00686E
|
| [12] |
Liu Z, et al 2020 Voltage issue of aqueous rechargeable metal-ion batteries Chem. Soc. Rev. 49 180-232 DOI: 10.1039/c9cs00131j
|
| [13] |
Chao D, Qiao S Z 2020 Toward high-voltage aqueous batteries: super- or low-concentrated electrolyte? Joule 4 1846-51 DOI: 10.1016/j.joule.2020.07.023
|
| [14] |
Yang Z, et al 2011 Electrochemical energy storage for green grid Chem. Rev. 111 3577-613 DOI: 10.1021/cr100290v
|
| [15] |
Ulaganathan M, et al 2016 Recent advancements in all-vanadium redox flow batteries Adv. Mater. Interfaces 3 1500309 DOI: 10.1002/admi.201500309
|
| [16] |
Wu M C, Zhao T S, Wei L, Jiang H R, Zhang R H 2018 Improved electrolyte for zinc-bromine flow batteries J. Power Sources 384 232-9 DOI: 10.1016/j.jpowsour.2018.03.006
|
| [17] |
Kolthoff I M, Tomsicek W J 1935 The oxidation potential of the system potassium ferrocyanide-potassium ferricyanide at various ionic strengths J. Phys. Chem. 4 945-54 DOI: 10.1021/j150367a004
|
| [18] |
Quan M, Sanchez D, Wasylkiw M F, Smith D K 2007 Voltammetry of quinones in unbuffered aqueous solution: reassessing the roles of proton transfer and hydrogen bonding in the aqueous electrochemistry of quinones J. Am. Chem. Soc. 129 12847-56 DOI: 10.1021/ja0743083
|
| [19] |
Delahay P, Pourbaix M, Van Rysselberghe P 1951 PotentialpH diagram of zinc and its applications to the study of zinc corrosion J. Electrochem. Soc. 98 101 DOI: 10.1149/1.2778110
|
| [20] |
Beh E S, et al 2017 A neutral pH aqueous organic-organometallic redox flow battery with extremely high capacity retention ACS Energy Lett. 2 639-44 DOI: 10.1021/acsenergylett.7b00019
|
| [21] |
Bard A J, Faulkner L R 2000 Electrochemical Methods: Fundamentals and ApplicationsNew YorkWiley
|
| [22] |
Zhou X, Lin L, Lv Y, Zhang X, Wu Q 2018 A Sn-Fe flow battery with excellent rate and cycle performance J. Power Sources 404 89-95 DOI: 10.1016/j.jpowsour.2018.10.011
|
| [23] |
Gong K, et al 2016 All-soluble all-iron aqueous redox-flow battery ACS Energy Lett. 1 89-93 DOI: 10.1021/acsenergylett.6b00049
|
| [24] |
Xie C, Duan Y, Xu W, Zhang H, Li X 2017 A low-cost neutral zinc-iron flow battery with high energy density for stationary energy storage Angew. Chem., Int. Ed. 56 14953-7 DOI: 10.1002/anie.201708664
|
| [25] |
Nicholson R S 1965 Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics Anal. Chem. 37 1351-5 DOI: 10.1021/ac60230a016
|
| [26] |
Hofmann J D, Schrder D 2019 Which parameter is governing for aqueous redox flow batteries with organic active material? Chem. Ing. Tech. 91 786-94 DOI: 10.1002/cite.201800162
|
| [27] |
Michibata H 2012 Vanadium: Biochemical and Molecular Biological Approaches. Vanadium: Biochemical and Molecular Biological ApproachesDordrechtSpringer 228 DOI: 10.1007/978-94-007-0913-3
|
| [28] |
Vijayakumar M, Wang W, Nie Z, Sprenkle V, Hu J 2013 Elucidating the higher stability of vanadium(V) cations in mixed acid based redox flow battery electrolytes J. Power Sources 241 173-7 DOI: 10.1016/j.jpowsour.2013.04.072
|
| [29] |
Li L, et al 2011 A stable vanadium redox-flow battery with high energy density for large-scale energy storage Adv. Energy Mater. 1 394-400 DOI: 10.1002/aenm.201100008
|
| [30] |
Agarwal H, Florian J, Goldsmith B R, Singh N 2019 V2+/V3+ redox kinetics on glassy carbon in acidic electrolytes for vanadium redox flow batteries ACS Energy Lett. 4 2368-77 DOI: 10.1021/acsenergylett.9b01423
|
| [31] |
Surez D J, et al 2014 Graphite felt modified with bismuth nanoparticles as negative electrode in a vanadium redox flow battery ChemSusChem 7 914-8 DOI: 10.1002/cssc.201301045
|
| [32] |
Jiang H R, et al 2020 A high power density and long cycle life vanadium redox flow battery Energy Storage Mater. 24 529-40 DOI: 10.1016/j.ensm.2019.07.005
|
| [33] |
Tseng T-M, Huang R-H, Huang C-Y, Hsueh K-L, Shieu F-S, Kinetic A 2013 Study of the platinum/carbon anode catalyst for vanadium redox flow battery J. Electrochem. Soc. 160 A690-6 DOI: 10.1149/2.073304jes
|
| [34] |
Tsai H M, Yang S J, Ma C C M, Xie X 2012 Preparation and electrochemical activities of iridium-decorated graphene as the electrode for all-vanadium redox flow batteries Electrochim. Acta 77 232-6 DOI: 10.1016/j.electacta.2012.05.099
|
| [35] |
Kim K J, et al 2012 Novel catalytic effects of Mn3O4 for all vanadium redox flow batteries Chem. Commun. 48 5455-7 DOI: 10.1039/c2cc31433a
|
| [36] |
Ejigu A, Edwards M, Walsh D A 2015 Synergistic catalyst-support interactions in a graphene-Mn3O4 electrocatalyst for vanadium redox flow batteries ACS Catal. 5 7122-30 DOI: 10.1021/acscatal.5b01973
|
| [37] |
Thu Pham H T, Jo C, Lee J, Kwon Y 2016 MoO2 nanocrystals interconnected on mesocellular carbon foam as a powerful catalyst for vanadium redox flow battery RSC Adv. 6 17574-82 DOI: 10.1039/C5RA24626A
|
| [38] |
Zhou H, et al 2014 CeO2 decorated graphite felt as a high-performance electrode for vanadium redox flow batteries RSC Adv. 4 61912-8 DOI: 10.1039/C4RA12339E
|
| [39] |
Shen Y, et al 2014 Electrochemical catalytic activity of tungsten trioxide-modified graphite felt toward VO2+/VO2+ redox reaction Electrochim. Acta 132 37-41 DOI: 10.1016/j.electacta.2014.03.107
|
| [40] |
Shah A B, Wu Y, Joo Y L 2019 Direct addition of sulfur and nitrogen functional groups to graphite felt electrodes for improving all-vanadium redox flow battery performance Electrochim. Acta 297 905-15 DOI: 10.1016/j.electacta.2018.12.052
|
| [41] |
Li Y, Parrondo J, Sankarasubramanian S, Ramani V 2019 Impact of surface carbonyl- and hydroxyl-group concentrations on electrode kinetics in an all-vanadium redox flow battery J. Phys. Chem. C 123 6370-8 DOI: 10.1021/acs.jpcc.8b11874
|
| [42] |
Roe S, Menictas C, Skyllas-Kazacos M 2016 A high energy density vanadium redox flow battery with 3 M vanadium electrolyte J. Electrochem. Soc. 163 A5023-8 DOI: 10.1149/2.0041601jes
|
| [43] |
Kausar N, Mousa A, Skyllas-Kazacos M 2016 The effect of additives on the high-temperature stability of the vanadium redox flow battery positive electrolytes ChemElectroChem 3 276-82 DOI: 10.1002/celc.201500453
|
| [44] |
Mousa A, Skyllas-Kazacos M 2015 Effect of additives on the low-temperature stability of vanadium redox flow battery negative half-cell electrolyte ChemElectroChem 2 1742-51 DOI: 10.1002/celc.201500233
|
| [45] |
Zhang J, et al 2011 Effects of additives on the stability of electrolytes for all-vanadium redox flow batteries J. Appl. Electrochem. 41 1215-21 DOI: 10.1007/s10800-011-0312-1
|
| [46] |
Peng S, et al 2012 Influence of trishydroxymethyl aminomethane as a positive electrolyte additive on performance of vanadium redox flow battery Int. J. Electrochem. Sci. 7 2440-47
|
| [47] |
Peng S, et al 2012 Stability of positive electrolyte containing trishydroxymethyl aminomethane additive for vanadium redox flow battery Int. J. Electrochem. Sci. 7 4388-96
|
| [48] |
Li Q, Bai A, Xue Z, Zheng Y, Sun H 2020 Nitrogen and sulfur co-doped graphene composite electrode with high electrocatalytic activity for vanadium redox flow battery application Electrochim. Acta 362 137223 DOI: 10.1016/j.electacta.2020.137223
|
| [49] |
Bhushan M, Kumar S, Singh A K, Shahi V K 2019 High-performance membrane for vanadium redox flow batteries: cross-linked poly(ether ether ketone) grafted with sulfonic acid groups via the spacer J. Membr. Sci. 583 1-8 DOI: 10.1016/j.memsci.2019.04.028
|
| [50] |
Si J, Lv Y, Lu S, Xiang Y 2019 Microscopic phase-segregated quaternary ammonia polysulfone membrane for vanadium redox flow batteries J. Power Sources 428 88-92 DOI: 10.1016/j.jpowsour.2019.04.100
|
| [51] |
Yin C, Gao Y, Xie G, Li T, Tang H 2019 Three dimensional multi-physical modeling study of interdigitated flow field in porous electrode for vanadium redox flow battery J. Power Sources 438 227023 DOI: 10.1016/j.jpowsour.2019.227023
|
| [52] |
Tsushima S, Suzuki T 2020 Modeling and simulation of vanadium redox flow battery with interdigitated flow field for optimizing electrode architecture J. Electrochem. Soc. 167 020553 DOI: 10.1149/1945-7111/ab6dd0
|
| [53] |
Zeng Y K, Zhao T S, Zhou X L, Wei L, Ren Y X 2017 A novel iron-lead redox flow battery for large-scale energy storage J. Power Sources 346 97-102 DOI: 10.1016/j.jpowsour.2017.02.018
|
| [54] |
Manohar A K, et al 2016 A high efficiency iron-chloride redox flow battery for large-scale energy storage J. Electrochem. Soc. 163 A5118-25 DOI: 10.1149/2.0161601jes
|
| [55] |
Jayathilake B S, Plichta E J, Hendrickson M A, Narayanan S R 2018 Improvements to the coulombic efficiency of the iron electrode for an all-iron redox-flow battery J. Electrochem. Soc. 165 A1630-8 DOI: 10.1149/2.0451809jes
|
| [56] |
Hawthorne K L, Petek T J, Miller M A, Wainright J S, Savinell R F 2015 An investigation into factors affecting the iron plating reaction for an all-iron flow battery J. Electrochem. Soc. 162 A108-13 DOI: 10.1149/2.0591501jes
|
| [57] |
Holze R, Lechner M D 2007 Electrochemistry : Electrochemical Thermodynamics and KineticsBerlinSpringer
|
| [58] |
Beverskog B 1996 Revised diagrams for iron at 25-300 C Corros. Sci. 38 2121-35 DOI: 10.1016/S0010-938X(96)00067-4
|
| [59] |
Luo J, et al 2017 Unraveling pH dependent cycling stability of ferricyanide/ferrocyanide in redox flow batteries Nano Energy 42 215-21 DOI: 10.1016/j.nanoen.2017.10.057
|
| [60] |
Luo J, et al 2019 Unprecedented capacity and stability of ammonium ferrocyanide catholyte in pH neutral aqueous redox flow batteries Joule 3 149-63 DOI: 10.1016/j.joule.2018.10.010
|
| [61] |
Yee E L, Cave R J, Guyer K L, Tyma P D, Weaver M J 1979 A survey of ligand effects upon the reaction entropies of some transition metal redox couples J. Am. Chem. Soc. 101 1131-7 DOI: 10.1021/ja00499a013
|
| [62] |
Chen Y D, Santhanam K S V, Bard A J 1981 Solution redox couples for electrochemical energy storage: I. Iron (III)iron (II) complexes with Ophenanthroline and related ligands J. Electrochem. Soc. 128 1460-7 DOI: 10.1149/1.2127663
|
| [63] |
Weber A Z, et al 2011 Redox flow batteries: a review J. Appl. Electrochem. 41 1137-64 DOI: 10.1007/s10800-011-0348-2
|
| [64] |
Waters S E, Robb B H, Marshak M P, Marshak M P 2020 Effect of chelation on iron-chromium redox flow batteries ACS Energy Lett. 5 1758-62 DOI: 10.1021/acsenergylett.0c00761
|
| [65] |
Lopez-Atalaya M, Codina G, Perez J R, Vazquez J L, Aldaz A 1992 Optimization studies on a Fe/Cr redox flow battery J. Power Sources 39 147-54 DOI: 10.1016/0378-7753(92)80133-V
|
| [66] |
Wang W, et al 2013 Recent progress in redox flow battery research and development Adv. Funct. Mater. 23 970-86 DOI: 10.1002/adfm.201200694
|
| [67] |
Gwynne E, Davies B H, Gwynne E 1952 The iodine-iodide interaction J. Am. Chem. Soc. I 2748-52
|
| [68] |
McIndoe J S, Tuck D G 2003 Studies of polyhalide ions in aqueous and non-aqueous solution by electrospray mass spectrometry Dalton Trans. 2003 244-8 DOI: 10.1039/b208035b
|
| [69] |
Zhao Y, Wang L, Byon H R 2013 High-performance rechargeable lithium-iodine batteries using triiodide/iodide redox couples in an aqueous cathode Nat. Commun. 4 1-7 DOI: 10.1038/ncomms2907
|
| [70] |
Li B, et al 2015 Ambipolar zinc-polyiodide electrolyte for a high-energy density aqueous redox flow battery Nat. Commun. 6 6303 DOI: 10.1038/ncomms7303
|
| [71] |
Li Z, Weng G, Zou Q, Cong G, Lu Y C 2016 A high-energy and low-cost polysulfide/iodide redox flow battery Nano Energy 30 283-92 DOI: 10.1016/j.nanoen.2016.09.043
|
| [72] |
Weng G M, Li Z, Cong G, Zhou Y, Lu Y C 2017 Unlocking the capacity of iodide for high-energy-density zinc/polyiodide and lithium/polyiodide redox flow batteries Energy Environ. Sci. 10 735-41 DOI: 10.1039/C6EE03554J
|
| [73] |
Mousavi M, et al 2020 Decoupled low-cost ammonium-based electrolyte design for highly stable zinc-iodine redox flow batteries Energy Storage Mater. 32 465-76 DOI: 10.1016/j.ensm.2020.06.031
|
| [74] |
Jang W J, Cha J S, Kim H, Yang J H 2021 Effect of an iodine film on charge-transfer resistance during the electro-oxidation of iodide in redox flow batteries ACS Appl. Mater. Interfaces 13 6385-93 DOI: 10.1021/acsami.0c22895
|
| [75] |
Yang J, Song Y, Liu Q, Tang A 2021 High-capacity zinc-iodine flow batteries enabled by a polymer-polyiodide complex cathode J. Mater. Chem. A 9 16093-8 DOI: 10.1039/D1TA03905A
|
| [76] |
Xie C, Liu Y, Lu W, Zhang H, Li X 2019 Highly stable zinc-iodine single flow batteries with super high energy density for stationary energy storage Energy Environ. Sci. 12 1834-9 DOI: 10.1039/C8EE02825G
|
| [77] |
Luo J, et al 2019 A 1.51 v pH neutral redox flow battery towards scalable energy storage J. Mater. Chem. A 7 9130-6 DOI: 10.1039/C9TA01469A
|
| [78] |
Zhang S, et al 2019 Recent progress in polysulfide redoxflow batteries Batter. Supercaps 2 627-37 DOI: 10.1002/batt.201900056
|
| [79] |
Gross M M, Manthiram A 2019 Long-life polysulfide-polyhalide batteries with a mediator-ion solid electrolyte ACS Appl. Energy Mater. 2 3445-51 DOI: 10.1021/acsaem.9b00253
|
| [80] |
Liu J, et al 2021 Sulfur-based aqueous batteries: electrochemistry and strategies J. Am. Chem. Soc. 143 15475-89 DOI: 10.1021/jacs.1c06923
|
| [81] |
Chen W, et al 2018 A manganese-hydrogen battery with potential for grid-scale energy storage Nat. Energy 3 428-35 DOI: 10.1038/s41560-018-0147-7
|
| [82] |
Chao D, et al 2019 An electrolytic Zn-MnO2 battery for high-voltage and scalable energy storage Angew. Chem., Int. Ed. 58 7823-8 DOI: 10.1002/anie.201904174
|
| [83] |
Xie C, et al 2020 A highly reversible neutral zinc/manganese battery for stationary energy storage Energy Environ. Sci. 13 135-43 DOI: 10.1039/C9EE03702K
|
| [84] |
Gong K, et al 2015 A zinc-iron redox-flow battery under 100 per kW h of system capital cost Energy Environ. Sci. 8 2941-5 DOI: 10.1039/C5EE02315G
|
| [85] |
Yao Y, Wang Z, Li Z, Lu Y-C 2021 A dendrite-free tin anode for high-energy aqueous redox flow batteries Adv. Mater. 33 2008095 DOI: 10.1002/adma.202008095
|
| [86] |
Huskinson B, et al 2014 A metal-free organic-inorganic aqueous flow battery Nature 505 195-8 DOI: 10.1038/nature12909
|
| [87] |
Yang B, Hoober-Burkhardt L, Wang F, Surya Prakash G K, Narayanan S R 2014 An inexpensive aqueous flow battery for large-scale electrical energy storage based on water-soluble organic redox couples J. Electrochem. Soc. 161 A1371-80 DOI: 10.1149/2.1001409jes
|
| [88] |
Er S, Suh C, Marshak M P, Aspuru-Guzik A 2015 Computational design of molecules for an all-quinone redox flow battery Chem. Sci. 6 885-93 DOI: 10.1039/C4SC03030C
|
| [89] |
Wedege K, Draevi E, Konya D, Bentien A 2016 Organic redox species in aqueous flow batteries: redox potentials, chemical stability and solubility Sci. Rep. 6 1-13 DOI: 10.1038/srep39101
|
| [90] |
Yang B, et al 2016 High-performance aqueous organic flow battery with quinone-based redox couples at both electrodes J. Electrochem. Soc. 163 A1442-9 DOI: 10.1149/2.1371607jes
|
| [91] |
Zhou W, et al 2020 Fundamental properties of TEMPO-based catholytes for aqueous redox flow batteries: effects of substituent groups and electrolytes on electrochemical properties, solubilities and battery performance RSC Adv. 10 21839-44 DOI: 10.1039/D0RA03424J
|
| [92] |
Nutting J E, Rafiee M, Stahl S S 2018 Tetramethylpiperidine N-oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-oxyl species: electrochemical properties and their use in electrocatalytic reactions Chem. Rev. 118 4834-85 DOI: 10.1021/acs.chemrev.7b00763
|
| [93] |
DeBruler C, et al 2017 Designer two-electron storage viologen anolyte materials for neutral aqueous organic redox flow batteries Chemistry 3 961-78 DOI: 10.1016/j.chempr.2017.11.001
|
| [94] |
Jin S, et al 2020 Near neutral pH redox flow battery with low permeability and long-lifetime phosphonated viologen active species Adv. Energy Mater. 10 2000100 DOI: 10.1002/aenm.202000100
|
| [95] |
Liu W, et al 2019 A highly stable neutral viologen/bromine aqueous flow battery with high energy and power density Chem. Commun. 55 4801-4 DOI: 10.1039/C9CC00840C
|
| [96] |
Liu T, Wei X, Nie Z, Sprenkle V, Wang W 2016 A total organic aqueous redox flow battery employing a low cost and sustainable methyl viologen anolyte and 4-HO-TEMPO catholyte Adv. Energy Mater. 6 1501449 DOI: 10.1002/aenm.201501449
|
| [97] |
Janoschka T, et al 2015 An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials Nature 527 78-81 DOI: 10.1038/nature15746
|
| [98] |
Tang H, et al 2021 Battery-everywhere design based on a cathodeless configuration with high sustainability and energy density ACS Energy Lett. 6 1859-68 DOI: 10.1021/acsenergylett.1c00555
|
| [99] |
Lei J, Yao Y, Wang Z, Lu Y C 2021 Towards high-areal-capacity aqueous zinc-manganese batteries: promoting MnO2 dissolution by redox mediators Energy Environ. Sci. 14 4418-26 DOI: 10.1039/D1EE01120K
|
| [100] |
Zheng X, et al 2021 Boosting electrolytic MnO2-Zn batteries by a bromine mediator Nano Lett. 21 8863-71 DOI: 10.1021/acs.nanolett.1c03319
|
| [101] |
Yadav G G, Turney D, Huang J, Wei X, Banerjee S 2019 Breaking the 2 V barrier in aqueous zinc chemistry: creating 2.45 and 2.8 V MnO2-Zn aqueous batteries ACS Energy Lett. 4 2144-6 DOI: 10.1021/acsenergylett.9b01643
|
| [102] |
Liu C, Chi X, Han Q, Liu Y 2020 A high energy density aqueous battery achieved by dual dissolution/deposition reactions separated in acid-alkaline electrolyte Adv. Energy Mater. 10 1903589 DOI: 10.1002/aenm.201903589
|
| [103] |
Zhong C, et al 2020 Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc-manganese dioxide batteries Nat. Energy 5 440-9 DOI: 10.1038/s41560-020-0584-y
|
| [104] |
Chao D, et al 2020 Atomic engineering catalyzed MnO2 electrolysis kinetics for a hybrid aqueous battery with high power and energy density Adv. Mater. 32 2001894 DOI: 10.1002/adma.202001894
|
| [105] |
Liang G, et al 2019 A universal principle to design reversible aqueous batteries based on deposition-dissolution mechanism Adv. Energy Mater. 9 1901838 DOI: 10.1002/aenm.201901838
|
| [106] |
Huang J, et al 2019 Low-cost and high safe manganese-based aqueous battery for grid energy storage and conversion Sci. Bull. 64 1780-7 DOI: 10.1016/j.scib.2019.09.020
|
| [107] |
Guo Z, et al 2020 An organic/inorganic electrode-based hydronium-ion battery Nat. Commun. 11 959 DOI: 10.1038/s41467-020-14748-5
|
| [108] |
Yan L, et al 2020 Solid-state proton battery operated at ultralow temperature ACS Energy Lett. 55 685-91 DOI: 10.1021/acsenergylett.0c00109
|
| [109] |
Wu T H, Lin Y Q, Althouse Z D, Liu N 2021 Dissolution-redeposition mechanism of the MnO2 cathode in aqueous zinc-ion batteries ACS Appl. Energy Mater. 4 12267-74 DOI: 10.1021/acsaem.1c02064
|
| [1] | Jing Lin, Mareen Schaller, Ruizhuo Zhang, Volodymyr Baran, Hao Liu, Ziming Ding, Sylvio Indris, Aleksandr Kondrakov, Torsten Brezesinski, Florian Strauss. High-entropy argyrodite glass-ceramic electrolytes for all-solid-state batteries[J]. Materials Futures, 2025, 4(2): 025105. DOI: 10.1088/2752-5724/adde76 |
| [2] | Lianfen Chen, Jiafan Fang, Jiexun Lin, Minying Zhao, Yiqing Liu, Jian-En Zhou, Yongbo Wu, Xiaoming Lin. The progress and promise for metal-organic framework-mediated synthesis of lithium-ion battery cathode materials[J]. Materials Futures. DOI: 10.1088/2752-5724/ade9e3 |
| [3] | Wenwen Sun, Yang Li, Chen Sun, Xuanyi Yuan, Haibo Jin, Yongjie Zhao. Deciphering the electrochemical-mechanical coupling failure mechanism of Na-NASICON solid-state batteries[J]. Materials Futures. DOI: 10.1088/2752-5724/adeff9 |
| [4] | Siyu An, Leonhard Karger, Sören L Dreyer, Yang Hu, Eduardo Barbosa, Ruizhuo Zhang, Jing Lin, Maximilian Fichtner, Aleksandr Kondrakov, Jürgen Janek, Torsten Brezesinski. Improving cycling performance of the NaNiO2 cathode in sodium-ion batteries by titanium substitution[J]. Materials Futures, 2024, 3(3): 035103. DOI: 10.1088/2752-5724/ad5faa |
| [5] | Huanbin Zheng, Jun Zeng, Xuanhong Wan, Xin Song, Chenxi Peng, Jiarui Wang, Luyi Sun, Hui Wang, Min Zhu, Jun Liu. ICE optimization strategies of hard carbon anode for sodium-ion batteries: from the perspective of material synthesis[J]. Materials Futures, 2024, 3(3): 032102. DOI: 10.1088/2752-5724/ad5d7f |
| [6] | Nohjoon Lee, Jihoon Oh, Jang Wook Choi. Anode-less all-solid-state batteries: recent advances and future outlook[J]. Materials Futures, 2023, 2(1): 013502. DOI: 10.1088/2752-5724/acb3e8 |
| [7] | Chenxi Zheng, Shijun Tang, Fangmei Wen, Jinxue Peng, Wu Yang, Zhongwei Lv, Yongmin Wu, Weiping Tang, Zhengliang Gong, Yong Yang. Reinforced cathode-garnet interface for high-capacity all-solid-state batteries[J]. Materials Futures, 2022, 1(4): 045103. DOI: 10.1088/2752-5724/aca110 |
| [8] | Ruijia Liu, Na Li, Enyue Zhao, Jinkui Zhao, Lingxu Yang, Wenjun Wang, Huijun Liu, Chaoliu Zeng. Facile molten salt synthesis of carbon-anchored TiN nanoparticles for durable high-rate lithium-ion battery anodes[J]. Materials Futures, 2022, 1(4): 045102. DOI: 10.1088/2752-5724/ac9cf7 |
| [9] | Marie-Claude Bay, Rabeb Grissa, Konstantin V Egorov, Ryo Asakura, Corsin Battaglia. Low Na- |
| [10] | Liubin Ben, Jin Zhou, Hongxiang Ji, Hailong Yu, Wenwu Zhao, Xuejie Huang. Si nanoparticles seeded in carbon-coated Sn nanowires as an anode for high-energy and high-rate lithium-ion batteries[J]. Materials Futures, 2022, 1(1): 015101. DOI: 10.1088/2752-5724/ac3257 |
| 1. | Chen, E., Sheng, Z., Sun, Y. et al. Sodium bicarbonate-assisted construction of honeycomb-like porous carbon materials with interconnected cavities for enhanced zinc ion storage. Colloids and Surfaces A Physicochemical and Engineering Aspects, 2025. DOI:10.1016/j.colsurfa.2025.136983 |
| 2. | Huang, L., Gu, J., Wang, B. et al. Surface pyrolysis towards graphite heterojunctions for aqueous Zinc-ion capacitor. Chemical Engineering Journal, 2025. DOI:10.1016/j.cej.2025.163094 |
| 3. | Liu, Y., Han, S.-G., Li, X. et al. Manganese dioxide cathode materials for aqueous zinc ion battery: Development, challenges and strategies. Energychem, 2025, 7(3): 100152. DOI:10.1016/j.enchem.2025.100152 |
| 4. | Huo, X., Shi, X., Wang, Q. et al. High-voltage pH-decoupling aqueous redox flow batteries for future energy storage. Current Opinion in Electrochemistry, 2025. DOI:10.1016/j.coelec.2024.101633 |
| 5. | Cao, F., Shan, X., Wu, J. et al. Toward Suppressing Hydrogen Evolution with Enhanced Performance for Bi-Modified NaTi2(PO4)3 Anodes in Aqueous Na-Ion Batteries. Batteries and Supercaps, 2025. DOI:10.1002/batt.202400767 |
| 6. | Cai, L., Zhang, Y., Yuan, W. et al. Ion-Specific Acetate-Mn2+ Coordination for Accelerating Desolvation Kinetics in Aqueous Mn-Ion Battery. Advanced Energy Materials, 2025. DOI:10.1002/aenm.202501026 |
| 7. | Abdullah, H., Shuwanto, H., Ginting, R.T. et al. Zn-Doped MnO2 microflake pseudocapacitive cathode on Ni(OH)2/Ni-Foam substrate as a promising charge storage material. Materials Science and Engineering B, 2024. DOI:10.1016/j.mseb.2024.117419 |
| 8. | Yang, H., Qi, Y., Wang, Z. et al. Sodium Nitrate/Formamide Deep Eutectic Solvent as Flame-Retardant and Anticorrosive Electrolyte Enabling 2.6 V Safe Supercapacitors with Long Cyclic Stability. Energy and Environmental Materials, 2024, 7(3): e12641. DOI:10.1002/eem2.12641 |
| 9. | Li, X., Xu, W., Zhi, C. Halogen-powered static conversion chemistry. Nature Reviews Chemistry, 2024, 8(5): 359-375. DOI:10.1038/s41570-024-00597-z |
| 10. | Zheng, Z., Ren, D., Li, Y. et al. Self-Assembled Robust Interfacial Layer for Dendrite-Free and Flexible Zinc-Based Energy Storage. Advanced Functional Materials, 2024, 34(17): 2312855. DOI:10.1002/adfm.202312855 |
| 11. | Li, X., Wang, S., Zhang, D. et al. Perovskite Cathodes for Aqueous and Organic Iodine Batteries Operating Under One and Two Electrons Redox Modes. Advanced Materials, 2024, 36(4): 2304557. DOI:10.1002/adma.202304557 |
| 12. | Zhang, H., Jiang, X., Wu, S. et al. Environmentally Friendly and Self-Healable Supercapacitors Realized by a NaCl-Penetrable Polyampholyte Conductive Hydrogel. ACS Applied Energy Materials, 2024, 7(2): 499-507. DOI:10.1021/acsaem.3c02440 |
| 13. | Li, P., Li, X., Guo, Y. et al. Development of an energy-dense and high-power Li-Cl2 battery using reversible interhalogen bonds. Chem, 2024, 10(1): 352-364. DOI:10.1016/j.chempr.2023.09.021 |
| 14. | Zhang, M., Su, Y., Li, G. et al. One-Pot preparation of microporous-polymer protected 3D porous Zn anode to enable advanced aqueous zinc batteries. Journal of Power Sources, 2024. DOI:10.1016/j.jpowsour.2023.233755 |
| 15. | Ke, X., Li, L., Wang, S. et al. Mn-oxide cathode material for aqueous Zn-ion battery: structure, mechanism, and performance. Next Energy, 2024. DOI:10.1016/j.nxener.2023.100095 |
| 16. | Wen, Q., Fu, H., Huang, Y.-D. et al. Constructing defect-free zincophilic organic layer via ultrasonic coating for anticorrosive and dendrite-free zinc anode. Nano Energy, 2023. DOI:10.1016/j.nanoen.2023.108810 |
| 17. | Xia, Y., Hong, Z., Zhou, L. et al. Multiscale simulations of surface adsorption characteristics of amino acids on zinc metal anode. Journal of Energy Chemistry, 2023. DOI:10.1016/j.jechem.2023.08.002 |
| 18. | Xu, B., Chen, J., Ding, Z. et al. The Progress and Outlook of Multivalent-Ion-Based Electrochromism. Small Science, 2023, 3(11): 2300025. DOI:10.1002/smsc.202300025 |
| 19. | Cai, L., Lu, L., Lan, Y. et al. Multidentate Chelation Enables High-Efficiency Mn2+ Storage in Polyimide Covalent Organic Framework for Aqueous All Mn-Ion Battery. Advanced Energy Materials, 2023, 13(37): 2301631. DOI:10.1002/aenm.202301631 |
| 20. | Wang, W., Wu, J., Zeng, C. New construction of polypyrrole interphase layers to improve performance stability of NaTi2(PO4)3 anode for aqueous Na-ion batteries. Solid State Ionics, 2023. DOI:10.1016/j.ssi.2023.116259 |
| 21. | Zhang, F., Lan, Y., Li, R. et al. Boosting the rate performance of primary Li/CFx batteries through interlayer conductive network engineering. Journal of Materials Chemistry A, 2023, 11(37): 20187-20192. DOI:10.1039/d3ta04102f |
| 22. | Zhang, H., Yu, Y., Zuo, Y. et al. Freeze-resistant and robust gel electrolyte for flexible aluminum-air batteries. Ionics, 2023, 29(8): 3087-3096. DOI:10.1007/s11581-023-05066-z |
| 23. | Zheng, J., Liu, X., Zheng, Y. et al. AgxZny Protective Coatings with Selective Zn2+/H+ Binding Enable Reversible Zn Anodes. Nano Letters, 2023, 23(13): 6156-6163. DOI:10.1021/acs.nanolett.3c01706 |
| 24. | Barros, Á., Artetxe, B., Eletxigerra, U. et al. Systematic Approach to the Synthesis of Cobalt-Containing Polyoxometalates for Their Application as Energy Storage Materials. Materials, 2023, 16(14): 5054. DOI:10.3390/ma16145054 |
| 25. | Tareen, A.K., Khan, K., Iqbal, M. et al. Recent advance in two-dimensional MXenes: New horizons in flexible batteries and supercapacitors technologies. Energy Storage Materials, 2022. DOI:10.1016/j.ensm.2022.09.030 |
| 26. | Huang, Y., Lu, Q., Wu, D. et al. Flexible MXene films for batteries and beyond. Carbon Energy, 2022, 4(4): 598-620. DOI:10.1002/cey2.200 |